

Contact us
Department of Biomaterial Sciences
The University of Tokyo
1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan
Phone:+81 80-4154-1362
E-mail:akira-isogai[at]g.ecc.u-tokyo.ac.jp
Research Project
Fundamental and Application Research of Cellulose using Chemistry and Structural Analyses
Cellulose nanocomposites by supramolecular chemistry engineering
Increasing environmental concerns demand the replacement of petroleum with renewable, sustainable resources to produce biodegradable and carbon-neutral products. As a natural, abundant and versatile biopolymer, cellulose has long been used in traditional applications such as paper and textiles and is now emerging in advanced fields including energy storage, healthcare, food, cosmetics, and paints and emulsions. Supramolecular chemistry offers a powerful strategy for engineering cellulose nanocomposites through specific, directional, tunable and reversible non-covalent interactions between nanocellulose and matrix components to achieve certain mechanical, chemical and biological properties. In this Review, we present the multiscale supramolecular engineering of cellulose nanocomposites and their fabrication and processing into materials. We provide a material and structural perspective of how the mechanical, ionic, optical and thermal properties and the environmental degradability of these nanocomposites can be regulated through supramolecular chemistry. Finally, we discuss how these approaches might address circularity and environmental sustainability goals, and we highlight major challenges and future prospects in the field, calling for further attention on supramolecular chemistry engineering to maximize the potential of these materials.
(Chen et al., Nature Reviews Materials, 2025)
Determination of molar-mass parameters of TEMPO-oxidized pulps by SEC/MALLS/RI
Molar-mass (MM) and MM distributions of TEMPO-oxidized pulps (TEMPO: 2,2,6,6-tetramethylpiperidine-1-oxyl radical) measured under the same conditions as those of the original pulps are important for understanding the changes of MM parameters of cellulose molecules in pulps during TEMPO-catalyzed oxidation. The carboxy groups in TEMPO-oxidized pulps prepared from softwood and hardwood bleached kraft pulps were esterified in high yield by trimethylsilyl diazomethane using a newly established protocol, which enabled complete dissolution in 8% (w/w) lithium chloride/N,N-dimethylacetamide (LiCl/DMAc). A linear relationship was obtained between the carboxy contents (0.05–2.68 mmol/g) of TEMPO-oxidized pulps and the signal area ratio of methyl/C1 carbon atoms in solid-state 13C NMR spectra of the esterified products. Next, 1% (w/v) LiCl/DMAc solutions of the original pulps and TEMPO-oxidized and then esterified products were subjected to size-exclusion chromatography with refractive index and multi-angle static laser light scattering detection. Chromatographic elution patterns, MM plots, conformation plots, and related MM parameters of the pulps and esterified products were obtained with good reproducibility. The results showed that the changes of the degree of polymerization and its distribution for TEMPO-oxidized pulps can be investigated in terms of carboxy content, mass recovery ratio, and TEMPO-catalyzed oxidation conditions.
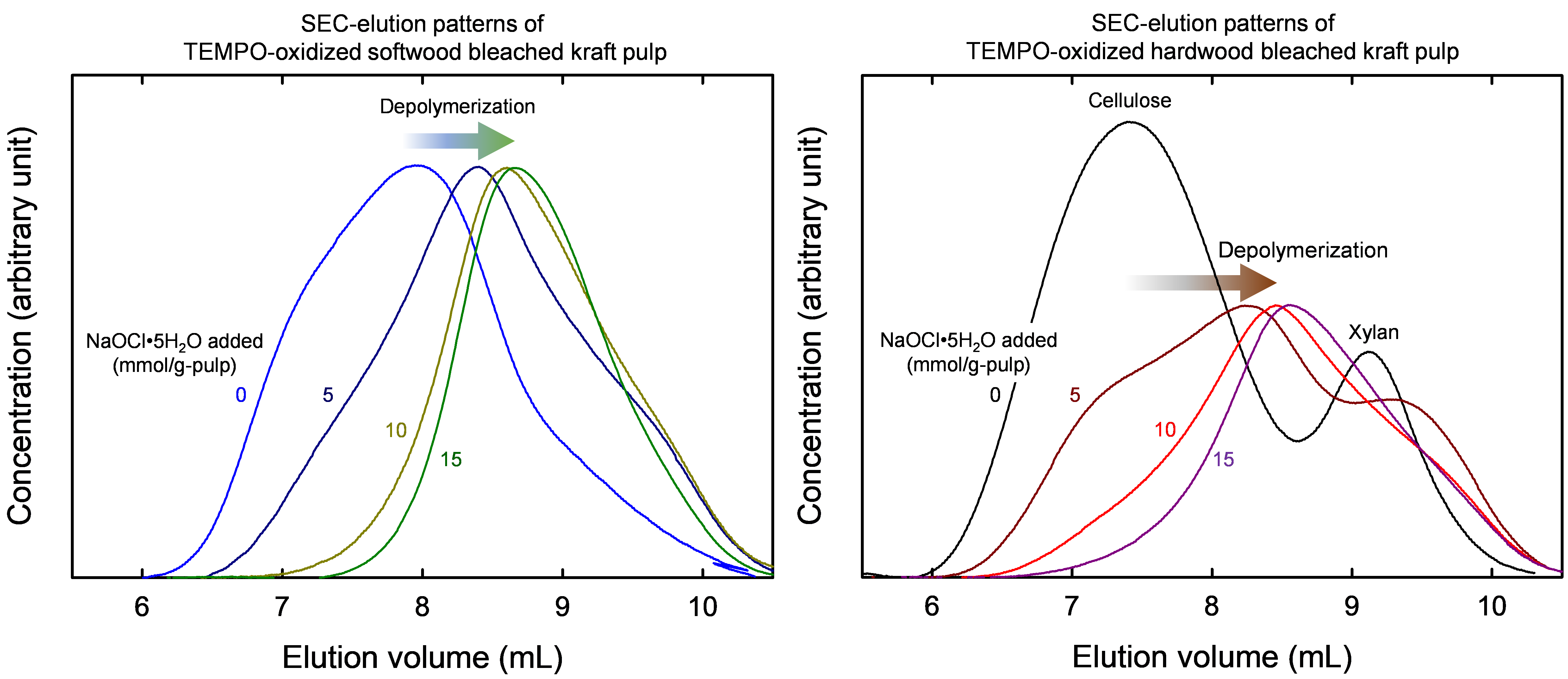
(Hou et al., Cellulose, 2025)
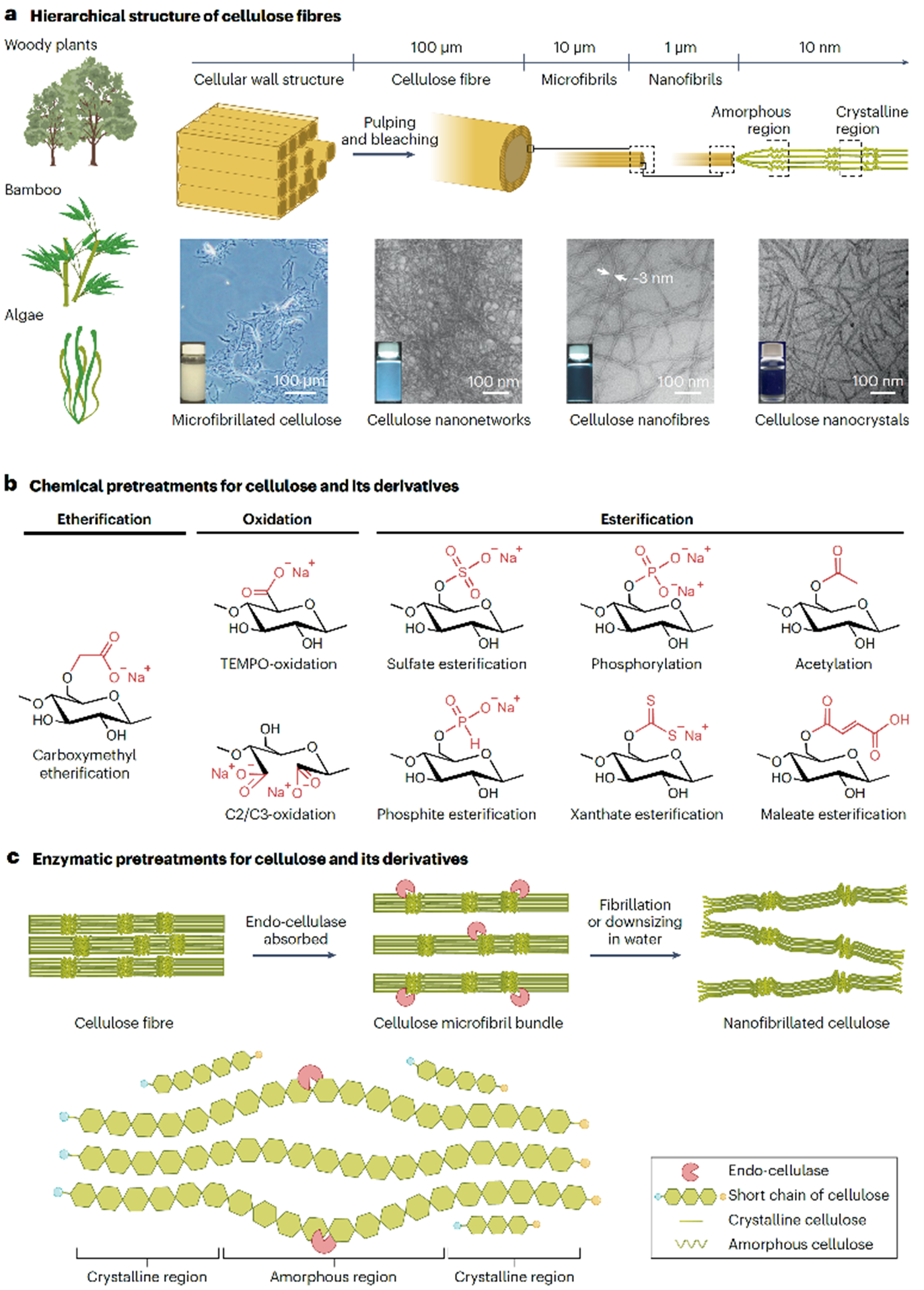
Nanocellulose
Nanocellulose is prepared from plant cellulose fibres, with or without chemical pretreatment, by mechanical disintegration in water through downsizing or fibrillation. When plant cellulose fibres are mechanically disintegrated in water without any chemical pretreatment, heterogeneous cellulose nanonetwork (CNNeW) structures are formed. When chemically pretreated plant cellulose fibres are mechanically disintegrated in water under suitable conditions, both CNNeWs and completely individualised cellulose nanofibres (or nanofibrils) (CNFs) or cellulose nanocrystals (CNCs) are obtained, depending on the nanofibrillation conditions. CNFs and CNCs have long and short average lengths of >200 and ≤200 nm, respectively. Another method of preparing nanocellulose is the biosynthesis of bacterial cellulose from low-molar-mass compounds. The product is called bacterial nanocellulose. Nanocellulose materials prepared from wood cellulose fibres are promising bio-based nanomaterials and are prepared from abundant and reproducible wood biomass resources. Chemical pretreatment enables the formation of dense concentrations of anionically charged groups in selected positions on the crystalline cellulose microfibril surfaces of each wood cellulose fibre. These chemically modified cellulose fibres can be completely nanodispersed in water by gentle mechanical disintegration under suitable conditions to form homogeneous CNFs and CNCs with widths as small as ~3 nm. Small widths and the presence of dense concentrations of anionically charged groups on each nanocellulose element are characteristics of chemically pretreated CNFs and CNCs. Diverse counterion exchanges between these CNFs or CNCs and various metal or alkylammonium ions are possible. These counterion-exchanged CNFs and CNCs have high water and moisture resistance, biodegradable/stable switching properties, antibacterial and superdeodorant properties, catalytic behaviour, and efficient hydrophilic/hydrophobic switching functionalities.
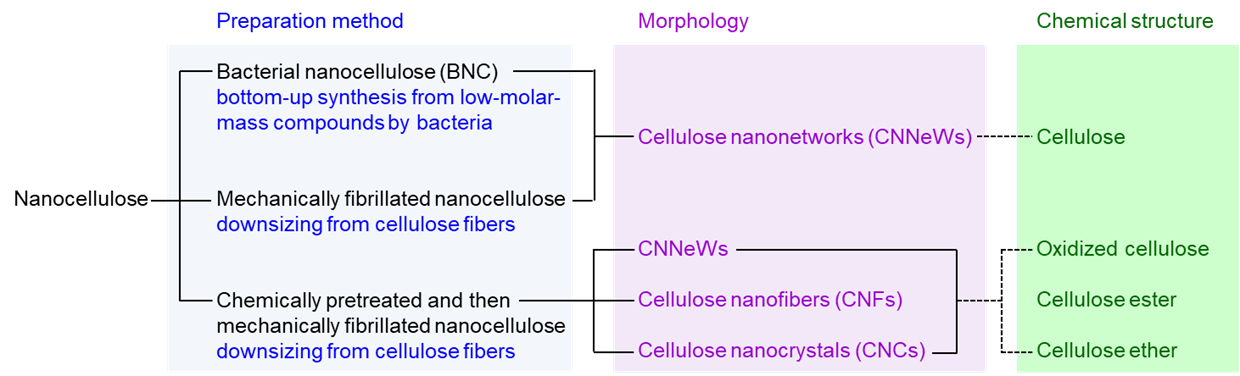
(Isogai, Polymers, Materials, and Technology, 2025)
Improvement of the durability of hydrogenated nitrile butadiene rubber sheets by adding phosphorylated cellulose nanofibers and metal (meth)acrylate
Nanocellulose materials are expected to partly replace petroleum-derived carbon black in rubber composites for the short-term improvement of their properties. In this study, phosphorylated cellulose nanofibers (P-CNFs) and hydrogenated nitrile-butadiene rubber (H-NBR) were oven-dried with or without metal (meth)acrylates, and the once-dried mixtures were kneaded and pressed to prepare cross-linked H-NBR/P-CNF/metal (meth)acrylate composite sheets. Neat H-NBR and carbon-black-containing H-NBR composite sheets were prepared as references. In addition to the tensile and dynamic thermomechanical properties, the, hardness, abrasion resistance, degree of hysteresis loss, and hot water/organic solvent resistance of the composite sheets were evaluated. The properties of the P-CNF containing composite sheets were better than those of the neat H-NBR sheet and carbon-black-containing composite sheets at the same P-CNF or carbon black content. The H-NBR/P-CNF/zinc methacrylate composite sheets showed the best performance in terms of not only the above properties, but also the hot water resistance, tensile properties, and abrasion resistance.
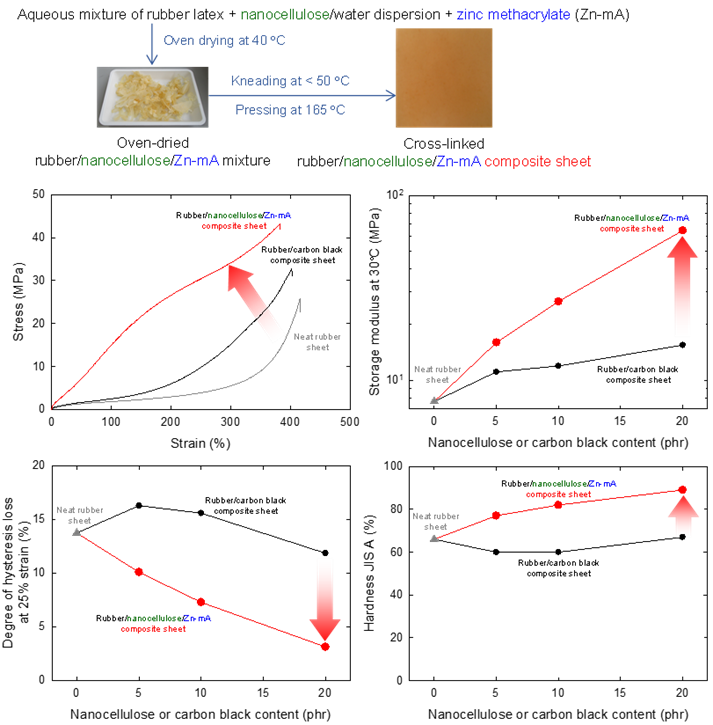
(Noguchi et al., Polymer Degradation and Stability, 2024)
Water repellency of cotton knitted fabrics treated with alkyl ketene dimers
Water repellency is one of the expected functions for originally hydrophilic cotton fabrics (CFs) in various applications. Aqueous dispersions of alkylketene dimers (AKDs) have been used as efficient sizing chemicals in practical papermaking. In the present study, we soaked CFs in weakly cationic AKD dispersions of various AKD concentrations, squeezed them, and cured/dried the AKD dispersion-containing wet CFs at 120 °C for 10 min. Scanning electron microscopy revealed flake-like AKD structures in the AKD-treated and air-dried CFs, which mostly disappeared when the AKD-related compounds (such as the original AKD, hydrolyzed AKD, and cellulose-reacted AKD molecules present in the CFs) melted and spread during curing. The contents of the AKD-related compounds in the CFs were determined by Fourier-transform infrared spectroscopy. When a dispersion with an AKD concentration of 6.7 g/L and a pH of 4.5 was used, the cured and dried CF contained ~0.49% AKD-related compounds. The resulting CF sample had prolonged water-absorption times (a measure of water repellency) of > 1800 s, even after 30 cycles of laundry treatment. The content of AKD-related compounds was decreased markedly from 0.49% to 0.06% and 0.01% by laundry treatment once and 30 times, respectively. Quite small amounts of AKD-related compounds remained in the CF after laundry treatment, and contributed to its high-water repellency. Therefore, the AKD treatment developed in the present study offers a practical and efficient means of conferring high water repellency on CFs.
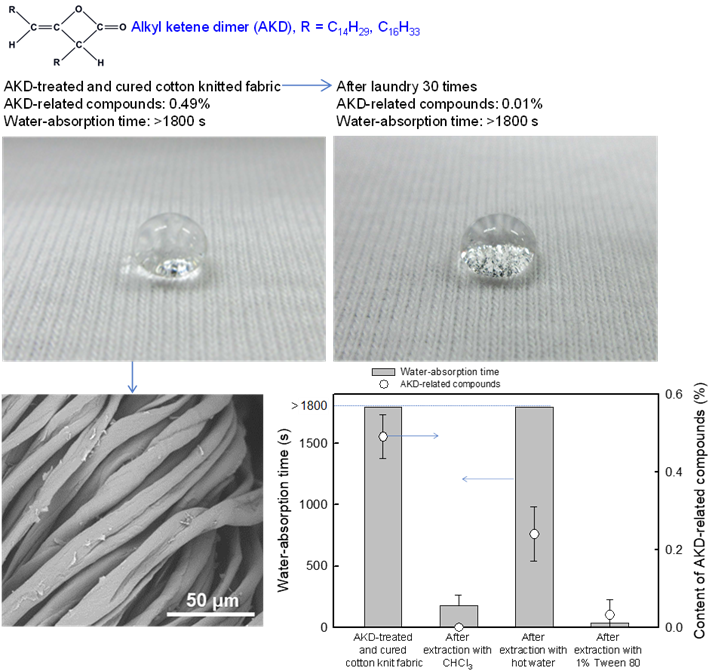
(Onodera et al., Cellulose, 2024)
Preparation and characterization of nanocellulose-reinforced water-soluble cellulose acetate films
A water-soluble cellulose acetate (CA) with a degree of acetyl substitution of 0.9 was dissolved in water and mixed with aqueous dispersions of reinforcing cellulose nanofibers (CNFs) at various molar ratios. CA/CNF composite films with CNF contents of 0%−16% were prepared by casting and drying the mixtures to improve the fundamental properties of the films. Aqueous dispersions of TEMPO-CNFs containing sodium carboxylate and protonated carboxy groups were separately prepared and used to produce CA composite films (TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl radical). Highly transparent CA/TEMPO-CNF films with tensile strengths and Young’s moduli more than twofold those of the 100% CA film were obtained at a TEMPO-CNF content of 16%. Transmission electron microscopy images of the film cross-sections showed that the TEMPO-CNFs were almost homogeneously and individually distributed in the CA/TEMPO-CNF-COOH composite films. The thermal expansion patterns of the 100% CA films were characteristically wavy between 30 and 100 °C, and the thermal expansion ratios decreased as the TEMPO-CNF contents of the films increased. The obtained results indicate that CA/CNF mixtures can be used as water-based coatings and paints with favorable properties.
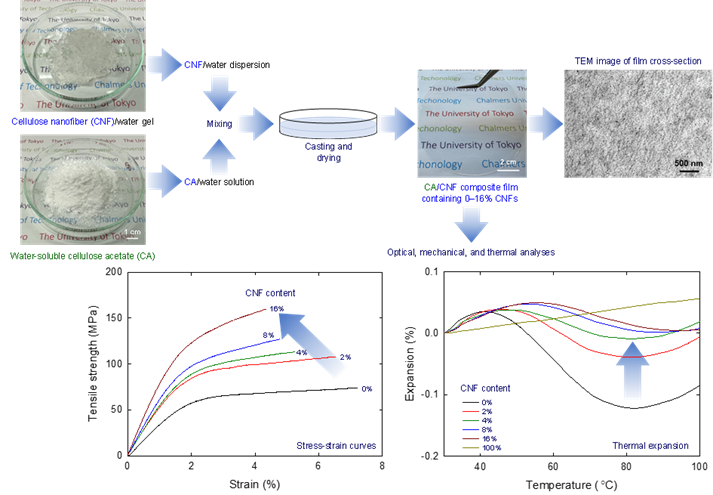
(Chen et al., Reactive and Functional Polymers, 2024)
Amidation of carboxy groups in TEMPO-oxidized cellulose for improving surface-hydrophobization and thermal stability of TEMPO-CNCs
Surface-hydrophobized cellulose nanomaterials (CNs) with high thermal degradation points are required for preparing various materials, such as epoxy nanocomposites, which possess high mechanical strength, optical transparency, and thermal stability. Amidation of carboxy groups in CNs is one possible chemical modification for hydrophilic CNs that contain abundant carboxy groups. However, achieving efficient amidation of high ratios of carboxy groups in CNs is highly challenging for industrial applications. In this study, carboxy group-containing fibrous wood pulp was subjected to amidation in heterogeneous solid/liquid systems to prepare products with high amidation ratios and high yields, while implementing cost-effective isolation and purification processes. Consequently, a partially acid-hydrolyzed wood pulp with abundant carboxy groups was first prepared. Subsequently, 88% and 91% of the carboxy groups in the pulp were successfully amidated using polyalkylene glycols-NH2 and octylamine, respectively. This was achieved by utilizing 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride and N-methylmorpholine as the condensation reagent and activator, respectively, in N,N-dimethylformamide (DMF) at approximately 23 °C for 16 h. The thermal degradation point increased from 224 °C for the acid-hydrolyzed pulp to over 250 °C after amidation. The amidated pulps were then converted into transparent dispersions, consisting of amidated cellulose nanocrystals, by homogenization in an epoxy monomer/DMF mixture using high-pressure homogenization.
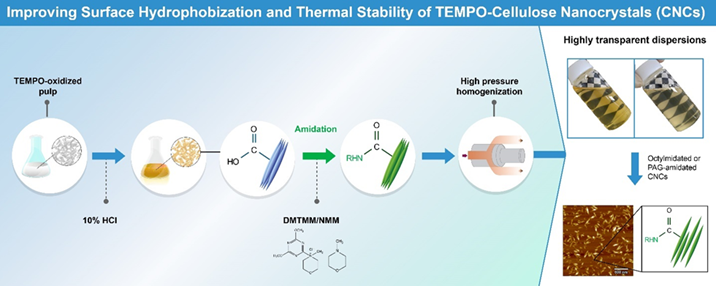
(Yoshikawa et al., Carbohydrate Polymers, 2024)
β-(1→4)-Polyglucuronic acids with C2/C3-ketones prepared from regenerated cellulose by catalytic oxidation using solid NaOCl·5H2O
Three commercial regenerated cellulose samples were subjected to TEMPO-catalyzed oxidation using solid NaOCl·5H2O as the primary oxidant for structural analyses of the oxidized products (TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl). The regenerated cellulose/water slurries became transparent solutions after oxidation for 60 min. The yields of the oxidized products were almost 100% when they were isolated as precipitates in ethanol/water mixtures. The solution-state NMR spectra revealed that the oxidized products were almost pure water-soluble β-(1→4)-polyglucuronic acids; the reaction conditions described herein ensured the complete oxidation of the C6‒OH groups in the regenerated cellulose samples to C6-carboxy groups. However, the solid-state 13C-NMR spectra of the oxidized products indicated that C2/C3-ketones (<20% of the total units) were formed during side reactions, which is characteristic for oxidized products prepared from regenerated cellulose with the C2/C3-glycol structure. These ketones were likely to form intermolecular hemiacetal linkages in the oxidized products. During conductivity titration of the oxidized products, it is necessary to control the sample masses to accurately determine the carboxy contents. The mass-average degree of polymerization decreased from 330‒890 for the original regenerated cellulose samples to 65‒79 for the oxidized products; substantial depolymerization is inevitable during TEMPO-catalyzed oxidation of the regenerated cellulose samples.
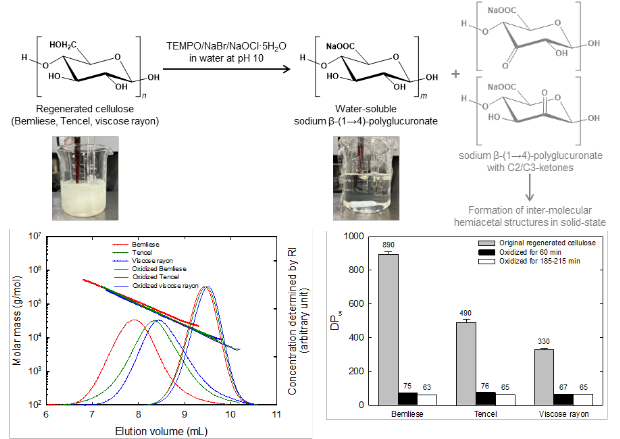
(Chitbanyong et al., Carbohydrate Polymers, 2024)
Improvement of water-resistance of natural rubber/nanocellulose composites
Natural rubber/cellulose nanofiber (NR/CNF) composites exhibit improved dry tensile properties compared with neat NR sheets. However, the presence of hydrophilic CNFs in the composites causes low water resistance, resulting in high water content and low tensile strength when the composites are soaked in water at 70°C for 7 d. Herein, water-resistant NR/CNF composites were developed by adding zinc methacrylate or aluminum acrylate, and the oven-dried NR/CNF/additive materials were counterion-exchanged with 0.1 M NH4OH. X-ray fluorescence analysis of the composite sheets indicates the reasons and mechanisms for the improved water resistance of the composites prepared with the tested additives by post-counterion exchange.
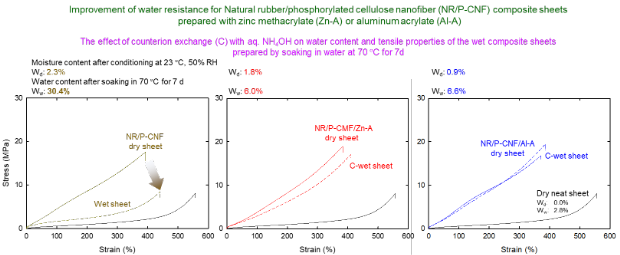
(Noguchi et al., Polymer Degradation and Stability, 2024)
Silicone oil/water Pickering emulsions with surface-hydrophobized cellulose nanofibers formed via in-situ homogenization with amino-silicone
The preparation of stable silicone oil/water emulsions is highly challenging because of the large difference in surface tension between the two liquids. Stable Pickering silicone oil/water emulsions were prepared by high-pressure homogenization of silicone oil/cellulose nanofibers/amino-silicone/water mixtures. TEMPO-oxidized cellulose nanofibers (TEMPO-CNFs) (TEMPO: 2,2,6,6-tetramethylpiperidine-1-oxyl radical) with protonated carboxy groups, TEMPO-CNF-COOH, nano-dispersed in water and amine groups in amino-silicone molecules were ionically linked in situ during homogenization treatment of the mixtures to form partly surface-hydrophobized TEMPO-CNFs by neutralization on the silicone oil droplet surfaces. More than 99% of the TEMPO-CNFs present in the systems were adsorbed on the silicone oil droplet surfaces in combination with amino-silicone and existed at the silicone oil/water interfaces. As a result, stable o/w Pickering emulsions with small and homogeneous volume mean-diameters (0.3–0.6 μm) were obtained, maintaining the droplet surfaces to have highly negative charges (–50 mV) owing to the dissociated carboxy groups of TEMPO-CNFs in water. When the as-prepared silicone oil Pickering emulsion was coated on glass plates and dried, transparent, water/moisture-resistant, and strongly water-repellent films were obtained. Thus, the Pickering emulsions prepared in this study are possibly used as coating or painting materials to continuously keep windows, glassware, and mirrors in a clean state.
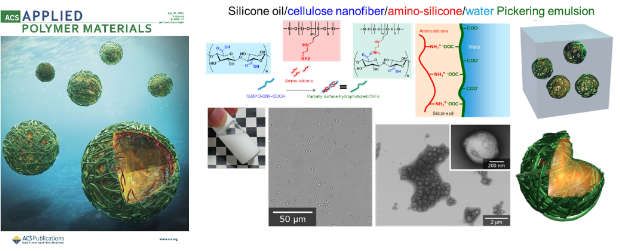
(Takeuchi et al., ACS Applied Polymer Materials, 2024)
Properties and structure of holocellulose nanofibers mechanically isolated from spent coffee grounds
Spent coffee grounds (SCGs) contain abundant polysaccharides of mannose (29%), galactose (11%), and glucose (11%) and are a promising source of holocellulose nanofibers (HCNFs). In this study, the mannan-rich HCNFs were isolated from the SCG holocellulose with the yield of 52 ± 3% SCGs using the ultrahigh-pressure wet jet mill. The HCNF refinement process was optimized by passing through a 95 μm nozzle 1–15 times. The 5-pass HCNFs were 2.4 nm widths, 0.7 μm lengths, 143 degrees of viscosity-average degrees of polymerization, and included mannan I crystals (5–10 nm) on the cellulose microfibrils. Hot water extraction (brewing) and/or lignification step likely allowed recrystallization of mannan using cellulose microfibrils as the substrate. The low crystallinity of HCNFs (16%) might be associated with the high crystalline mannan I. The redispersed HCNFs had 30-50 nm widths and were not fully fibrillated again into the original HCNFs morphologies but were comparable to mechanically milled CNFs. The SCGs-derived HCNFs has high potential for application in the food industry.

(Kanai et al., Carbohydrate Polymer Technologies and Applications, 2024)
Structural analyses of supernatant fractions in TEMPO-oxidized pulp/water reaction mixtures separated by centrifugation and dialysis
Side reactions occurring on cellulose during 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TMEPO)-catalyzed oxidation have not been considered to be significant. Then, TEMPO-oxidized hardwood and softwood bleached kraft pulps (HBKP and SBKP) were prepared with an excess NaOCl·5H2O. Supernatant fractions (SFs) were obtained in the aqueous reaction mixtures of TEMPO-oxidized pulps by centrifugation and dialysis. The SFs with carboxyl contents of 5.0 and 4.2 mmol/g were obtained in the yields of 19% and 30% from HBKP and SBKP, respectively. These carboxy contents are much higher than those (2.6–2.7 mmol/g) of the precipitate fractions in the TEMPO-oxidized pulps. Solid-state 13C-NMR spectra and other analyses revealed that the water-soluble β-(1→4)-polyglucuronic acids were predominantly present in the SFs. In addition, water-insoluble TEMPO-oxidized cellulose nanocrystals were present in the SFs, but they constituted less than ~10% of the SFs. The mass-average degrees of polymerization (DPw) of the SFs obtained from HBKP and SBKP were 166 and 155, respectively, whereas the original HBKP and SBKP had DPw values of 1990 and 2140, respectively. These substantial depolymerization and formation of the water-soluble β-(1→4)-polyglucuronic acids occur on cellulose and oxidized cellulose molecules as side reactions during TEMPO-catalyzed oxidation, which should be considered for structural analyses of TEMPO-oxidized products.
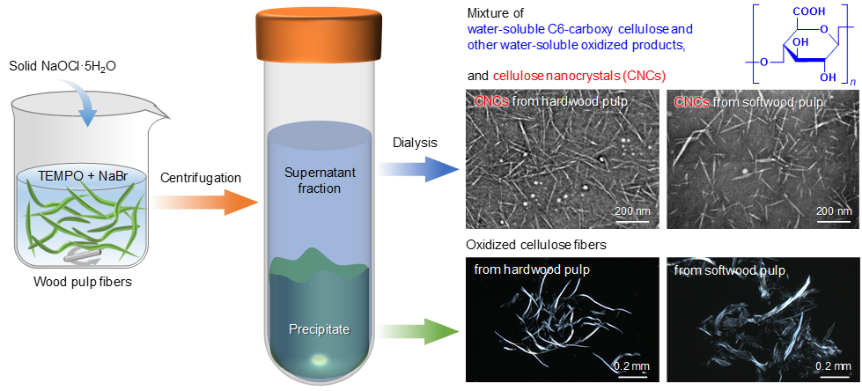
(Hou et al., Carbohydrate Polymers, 2024)
Acetylation of cotton knitted fabrics for improved quick drying after water absorption
Quick drying after water or sweat absorption is an important function of underwear. In this study, the hydroxy groups of cotton knitted fabrics (CFs) were partially acetylated, maintaining the original fabric structure. The following three heterogeneous acetylation processes were used: Ac-I (Ac2O/H2SO4/toluene), Ac-II (Ac2O/H2SO4/AcOH/water), and Ac-III (Ac2O/AcONa) systems (Ac2O, acetic anhydride; AcOH, acetic acid; AcONa, sodium acetate). Acetylated cotton knitted fabrics (AcCFs) with degree of substitution (DS) ≤0.5 and yields of >80% were prepared. AcCFs prepared with the Ac-III system gave high degree of polymerization (DP) values of >1500, whereas those prepared with the Ac-II system exhibited low DP values of ≤400. The moisture contents of AcCFs at 20°C and 65% relative humidity decreased from 7.1% to 4.7% with increasing DS value up to 0.46; introducing hydrophobic acetyl groups into the CFs decreased their hydrophilic nature. Quick drying similar to that of a polyester fabric was achieved for some of the AcCFs with DS values of <0.2. When the acetyl groups in the AcCFs were homogeneously distributed across each fiber width (achieved for AcCFs prepared with the Ac-II system), quick drying was evident in the AcCFs. The crystallinities and crystal widths of cellulose I for the AcCFs with DS values of ≤0.28 were almost unchanged compared with those of the original CFs. However, neither the crystallinities nor crystal widths of cellulose I were directly related to quick drying after water absorption. Thermal degradation of the AcCFs varied between the acetylation systems, and depended on the DP values and/or the presence of sulfate ester groups in the AcCFs.
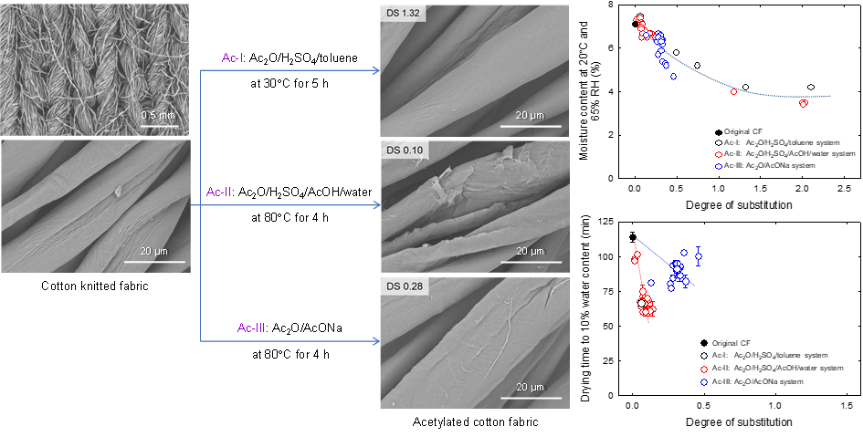
(Onodera et al., Cellulose, 2024)
Distribution of carboxy groups in TEMPO‑oxidized cellulose nanofibrils prepared from never‑dried Japanese cedar holocellulose, Japanese cedar‑callus, and bacterial cellulose
We prepared 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized samples from never-dried Japanese cedar (JC) holocellulose, JC-callus, and bacterial cellulose (BC). The original never-dried samples and their TEMPO-oxidized products were characterized by neutral sugar composition analysis. TEMPO-oxidized cellulose nanofibrils (TEMPO-CNFs) were prepared from the TEMPO-oxidized samples by ultrasonication in water. The carboxy groups in TEMPO-CNFs were position-selectively esterified with 9-anthryl diazomethane (ADAM) to prepare TEMPO-CNF-COOCH2-C14H9 samples, which had UV absorption peak at 365 nm. The mass-average degree of polymerization (DPw) values of 1% lithium chloride/N,N-dimethylacetamide (LiCl/DMAc) solutions of the original samples were determined by size-exclusion chromatography in combination with multi-angle laser-light scattering, ultra violet absorption, and refractive index detection (SEC/MALLS/UV/RI), and were 5490, 2660, and 2380 for the JC holocellulose, JC-callus, and BC samples, respectively. The TEMPO-CNF-COOCH2-C14H9 sample solutions in 1% LiCl/DMAc were analyzed by SEC/MALLS/UV/RI to obtain SEC elution patterns. The patterns corresponded to the molar mass and carboxy group distributions of the samples, which were detected by RI and UV absorption of anthryl groups, respectively. The carboxy groups existed in the entire molar mass distribution regions of all the TEMPO-CNF samples, although their lower molar mass regions contained higher carboxy group densities. The obtained results indicate that random depolymerization occurred on the cellulose microfibril surfaces at the initial stage of TEMPO-catalytic oxidation and/or ultrasonication in water. This depolymerization mechanism can explain all the obtained SEC-elution patterns of the TEMPO-CNFs, without considering the presence of periodically disordered regions in the cellulose microfibrils of the never-dried cellulose samples.
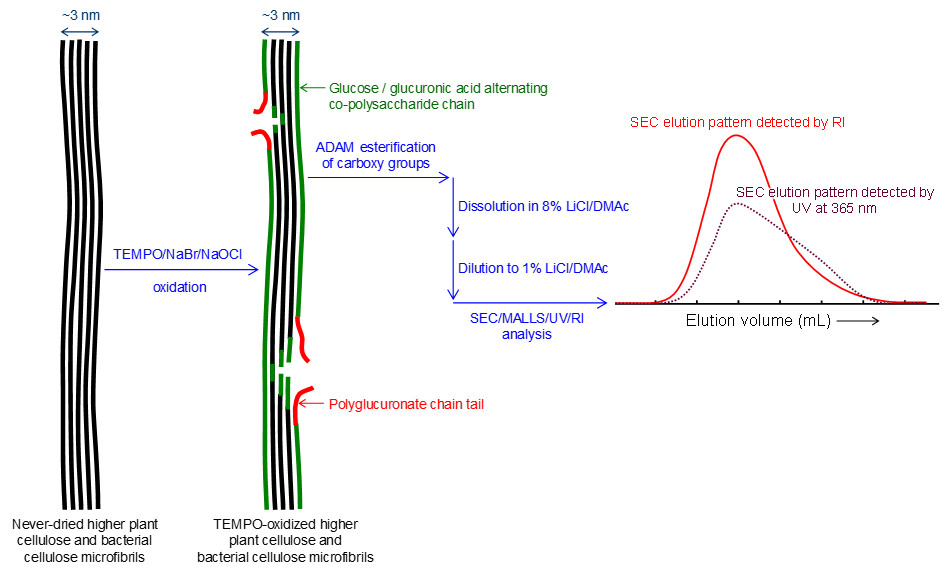
(Ono et al., Cellulose, 2024)
n-Type semiconductor with energy storage made from chitosan
Chitosan, obtained from crustaceans such as crabs and shrimp, has been mainly used in medicine; however, no studies on its use in electronic applications with semiconducting and large storage properties have been reported. Here, we report an n-type semiconducting biomaterial with energy storage properties of 694.4 mJ/m2 consisting of a chitosan nanofiber (ChNF) film with N-type negative resistance. The ChNF generates an alternative-current wave with a frequency of 7.8 MHz at a threshold voltage of 187 MV/m from a direct-current voltage source, with the switching effect of a third-order resistance change. This is due to the Gunn effect, caused by the repeated voltage-induced generation of a strong electric field domain (electric double layer) at the cathode and its disappearance at the anode of the ChNF device. Electron spin resonance spectral analysis showed that conducting electrons of the ChNF were identified as radicals on the aminyl radical, N⋅H. Paper electronics made from marine products are a great boon to a renewable society.
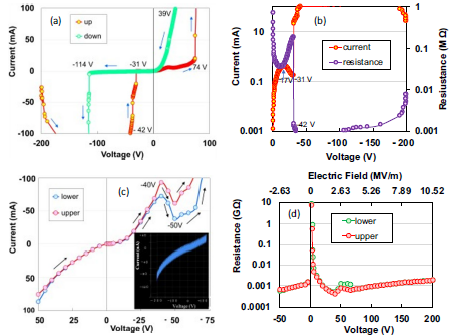
(Fukuhara et al., AIP Advances, 2024)
Effects of UV irradiation of TEMPO‑oxidized cellulose nanofibril/water dispersions on chemical structure, molar mass, and morphology
A commercial TEMPO -oxidized cellulose (TOC) and three laboratory-made TOC samples prepared under different conditions were converted to aqueous 0.4% dispersions of TEMPO-oxidized cellulose nanofibrils (TEMPO-CNFs) by high-pressure homogenization in water under the same conditions. The obtained TEMPO-CNF/water dispersions were irradiated with ultraviolet (UV) light for 0–48 h using a high-pressure mercury lamp at a main wavelength of 365 nm. The changes in the light transmittance, viscosity, pH, zeta-potential, and average particle size of the dispersions, and mass recovery ratio, molar mass, and carboxylate content of the acid-insoluble and freeze-dried fractions separated and isolated from the UV-irradiated dispersions were investigated. The results were analyzed in terms of the UV-irradiation time. The dispersion pH, and the mass recovery ratio, carboxylate content, and mass-average degree of polymerization (DPw) of the acid-soluble fraction decreased with increasing UV-irradiation time. This shows that some acid-soluble acidic compounds were formed from the TEMPO-CNFs via UV-induced depolymerization, degradation, and removal of carboxylate groups from the TEMPO-CNFs in water, depending on the UV-irradiation time. The average CNF length, which was determined from atomic force microscopy images, decreased with increasing UV-irradiation time. After UV irradiation of dispersions of the laboratory-made TEMPO-CNFs for 12 h, the average CNF lengths were 130–150 nm and their length distributions were narrower. UV irradiation of TEMPO-CNF/water dispersions is therefore a promising method for efficiently decreasing the dispersion viscosity, DPw, and average CNF length, depending on the TEMPO-CNF properties and UV-irradiation conditions.
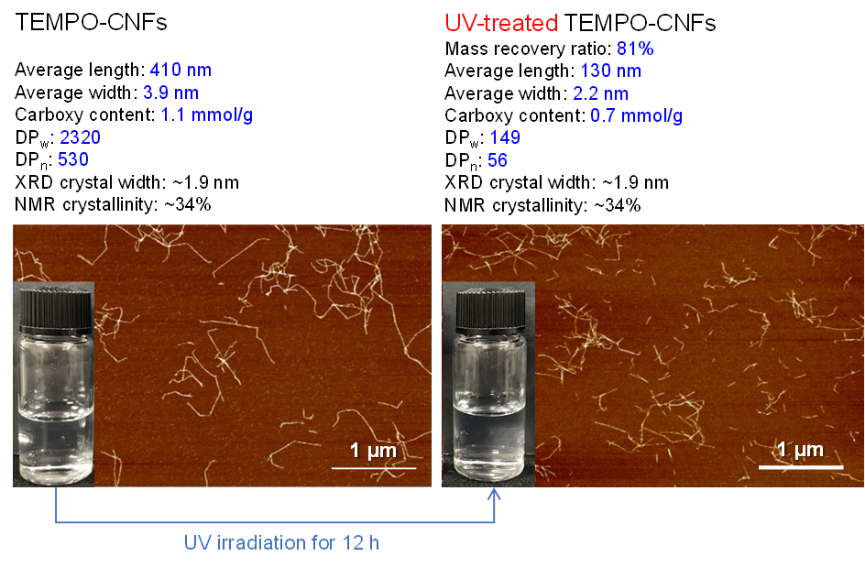
(Ning et al., Cellulose, 2024)
Polyglucuronic acids prepared from α-(1→3)-glucan by TEMPO-catalytic oxidation
2,2,6,6-Tetramethylpiperidine-1-oxyl radical (TEMPO)-catalytic oxidation was applied to a water-insoluble -(1→3)-glucan in water at pH 10 and room temperature (~24 °C), with solid NaOCl·5H2O as the primary oxidant. Oxidation with NaOCl at 15 mmol/g gave a water-soluble TEMPO-oxidized product at a mass recovery ratio of 97%. The carboxy content of the TEMPO-oxidized product was 5.3 mmol/g, which corresponds to a degree of C6-oxidation (DO) of 93%. A new water-soluble -(1→3)-polyglucuronic acid with a nearly homogeneous chemical structure was therefore quantitatively obtained. X-ray diffraction and solid-state 13C-NMR spectroscopic analyses showed that the original α-(1→3)-glucan and its TEMPO-oxidized product with a carboxy content of 5.3 mmol/g had crystalline structures, whereas the oxidized products with DOs of 50% and 66% had almost disordered structures. The carboxy groups in the oxidized products were regioselectively methyl esterified with trimethylsilyl diazomethane, and analyzed by using size-exclusion chromatography with multi-angle laser-light scattering and refractive index detections. The results show that the original α-(1→3)-glucan and its oxidized products with DOs of 50%, 66%, and 93% had weight-average degrees of polymerization of 671, 288, 54, and 45, respectively. Substantial depolymerization of the -(1→3)-glucan molecules therefore occurred during catalytic oxidation, irrespective of the oxidation pH.
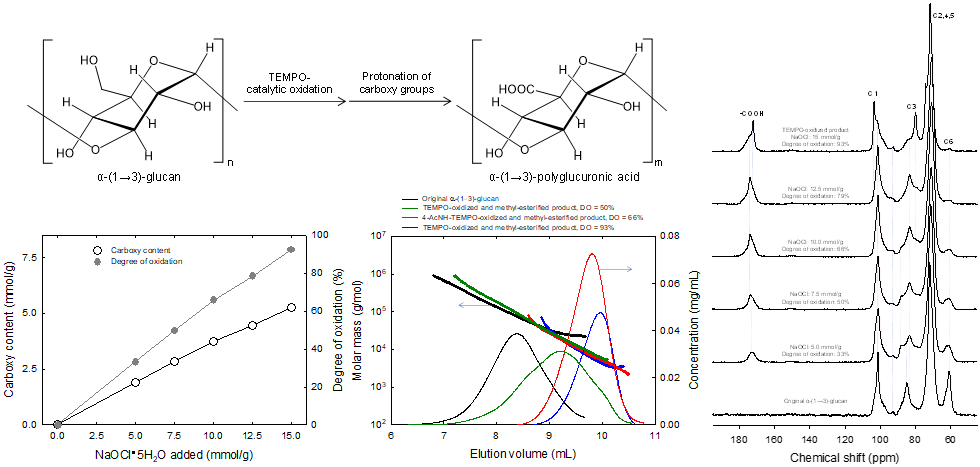
(Chitbanyong et al., Carbohydrate Polymers, 2024)
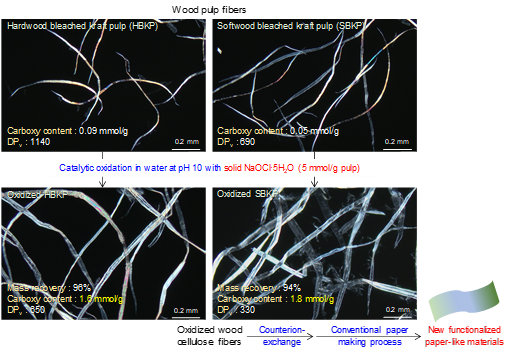
Comprehensive study of preparation of carboxy group-containing cellulose fibers from dry-lap kraft pulps by catalytic oxidation with solid NaOCl
Catalytic oxidation of commercial and dry-lap hardwood and softwood bleached kraft pulps (HBKP and SBKP, respectively) with TEMPO and NaBr has been comprehensively studied using solid NaOCl∙5H2O as the primary oxidant for preparation of carboxy group-containing cellulose fibers. When the amount of solid NaOCl added to the pulp suspension at pH 10 was 5 mmol/g pulp, the high initial reaction rate, a short time for complete oxidation, and high carboxy content and viscosity-average degree of polymerization for both the oxidized HBKP and SBKP were achieved. When they were prepared with 15 mmol NaOCl/g pulp, the mass recovery ratios of the water-insoluble oxidized cellulose fibers decreased to 40% and 26% for HBKP and SBKP, respectively, and their viscosity-average degrees of polymerization decreased to 180–230; side reactions always occurred on the pulps during oxidation. Solid-state 13C-NMR and X-ray diffraction showed that the crystallinity and crystal size of cellulose I in the original pulps were preserved for the oxidized cellulose fibers prepared with 2.5–15 mmol NaOCl/g pulp. The obtained carboxy group-rich cellulose fibers can be used as scaffolds for diverse cationic compounds, and are possible to be used as new functional group-containing sheet materials using the conventional papermaking process.
(Hou et al., ACS Sustainable Chemistry & Engineering, 2023)
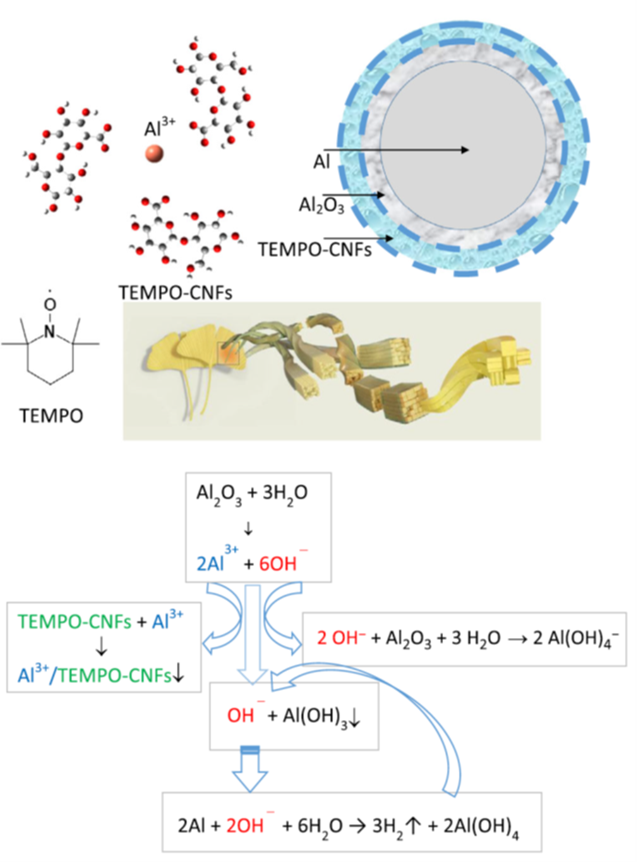
TEMPO-Oxidized Cellulose Nanofibers as Pseudocatalysts for in Situ and on-Demand Hydrogen Generation via Aluminum Powder/Pure Water Reactions at a Temperature below 50 °C
Cellulose nanofibers (CNFs) prepared via 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation of the C6 primary hydroxyls of native cellulose to carboxylates are used as pseudocatalysts for enhancing the aluminum powder/pure water reactions. The Al powder/pure water reaction is a stepwise reaction. It starts from hydration of the outmost native Al2O3 thin layer and then the reaction of the inner metallic Al with water. At lower temperatures (<50 °C), OH– and Al3+ ions are the preliminary products of the native Al2O3 thin layer hydration. Once Al powders are mixed with pure water containing 0.1–0.5 wt% TEMPOCNFs, condensed networks consisting of TEMPO-CNFs self-establish over the native Al2O3 thin layer. Al3+ ions are captured by TEMPO-CNFs via the formation of insoluble Al3+/TEMPO-CNFs complexing nanostructures and the conjugated OH– ions are being restricted nearby the native Al2O3-based thin layer via electrostatic repulsion. A highly alkaline condition (pH>11) is dynamically generated, and as a result, the native Al2O3 thin layer dissolves rapidly via the reaction with OH– ions. The OH– ions function also as catalysts, accelerating the reaction of metallic Al with water. Al powders (2–200 μm) react promptly and a nearly 100%Al/H2 conversion is obtained at the reaction temperature below 50°C.
(Fugetsu et al., Advanced Energy & Sustainability Research, 2023)
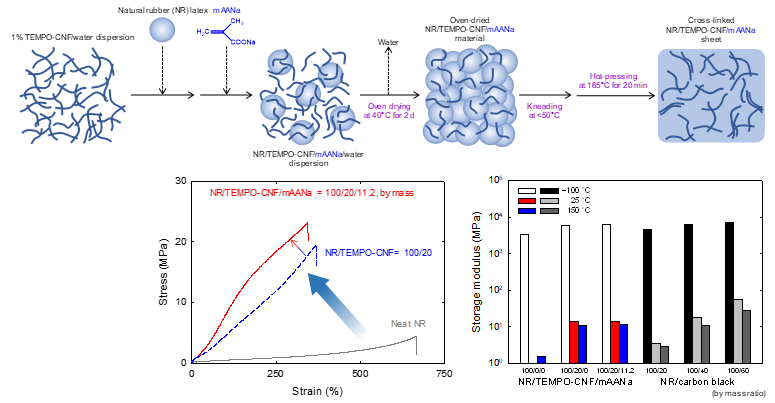
Natural rubber composites with high strength, modulus, water-resistance, and thermal stability, prepared with cellulose nanofibrils and sodium methacrylate
Natural rubber (NR) composite sheets consisting of TEMPO-oxidized cellulose nanofibrils (TEMPO-CNFs) and sodium methacrylate (mAANa) were prepared. An aqueous mixture of NR latex, TEMPO-CNFs, and mAANa was oven-dried; the dried material was kneaded to prepare cross-linked composite sheets. The NR/TEMPO-CNF/mAANa (100/20/11.2 by mass) sheets exhibited high tensile strengths of ~23 MPa, whereas the tensile strengths of the neat NR sheets were ~5 MPa. Scanning electron microscopy observations indicate that the TEMPO-CNFs formed fewer aggregates in the composite sheet containing mAANa than in the mAANa-free sheets. Thermomechanical tests indicate that the NR/TEMPO–CNF/mAANa sheets had higher storage moduli over the entire range from –100°C to 200°C than the neat NR sheet. The NR/TEMPO-CNF/mAANa composite sheets exhibited hysteresis loss and permanent deformation behaviors, similar to or better than those of NR/carbon black composite sheets. Thus, the NR/TEMPO-CNF/mAANa composite sheets can be used in a similar manner as those consisting of all-carbon neutral components.
(Noguchi et al., Composites Part A, 2023)
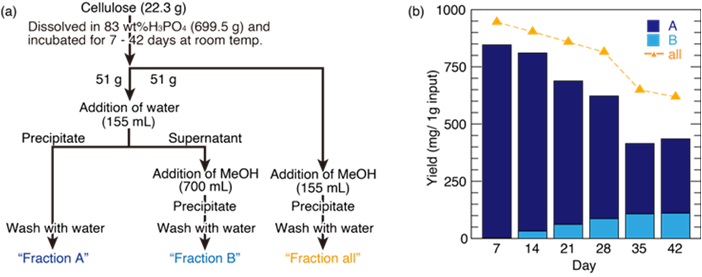
Quantitative analysis on the formation of monodisperse cello-oligomer obtained by phosphoric acid hydrolysis
Hydrolysis of cellulose in the concentrated phosphoric acid is known to give two distinctive mono-disperse cello-oligomers: the degree of polymerization (DP) of 7 and 15. To understand the formation mechanism of monodisperse cello-oligomers, the transition of DP during phosphoric hydrolysis was monitored by the size-exclusion chromatography combined with multiangle laser-light scattering analyses (SEC/MALLS). The obtained results suggested that not the hydrolysis at specific length but the random hydrolysis was likely. The rate of hydrolysis slowed down when the DP reached around 40, and the pool of cello-oligomers appeared at the incubation of 35 days. Then, the mono-disperse cello-oligomers were recovered from the pool of cello-oligomers by the fractionation based on the solubility difference.
(Isobe et al., Cellulose, 2023)
Molar masses and molar mass distributions of commercial regenerated cellulose materials and softwood dissolving pulp determined by SEC/MALLS
The molar masses and molar mass distributions of three commercial regenerated cellulose samples, viscose rayon, Tencel, and Bemliese (or cuprammonium nonwoven), have been determined by dissolution in 8% (w/w) lithium chloride/N,N-dimethylacetamide (LiCl/DMAc) and subsequent size-exclusion chromatography with multi-angle laser-light scattering detection (SEC/MALLS). Before dissolution in LiCl/DMAc, the regenerated cellulose samples were pretreated by the following three methods: (1) soaking in ethylene diamine (EDA) and subsequent solvent exchange to N,N-dimethylacetamide (DMAc) through methanol, (2) soaking in water and subsequent solvent exchange to DMAc through ethanol, and (3) soaking in water and subsequent solvent exchange to tert-butyl alcohol through ethanol and freeze dying. The pretreated samples were dissolved in 8% (w/w) LiCl/DMAc by stirring the cellulose/LiCl/DMAc mixtures for 1–3 weeks followed by dilution to 1% (w/v) LiCl/DMAc for SEC/MALLS analysis. The EDA- and water-pretreated samples gave almost the same SEC-elution pattens and molar mass plots, resulting in similar number- and mass-average molar masses. However, the freeze-dried samples gave 10%‒20% lower mass recovery ratios than those obtained for the EDA- or water-pretreated samples, probably because of incomplete dissolution of the freeze-dried samples in 8% (w/w) LiCl/DMAc. The average mass-average degree of polymerization values of viscose rayon, Tencel, and Bemliese were 340, 530, and 880, respectively. The slopes of the conformation plots were 0.58–0.62, showing that all of the molecules in the three regenerated cellulose samples were dissolved in 1% (w/v) LiCl/DMAc, forming linear random-coil conformations.
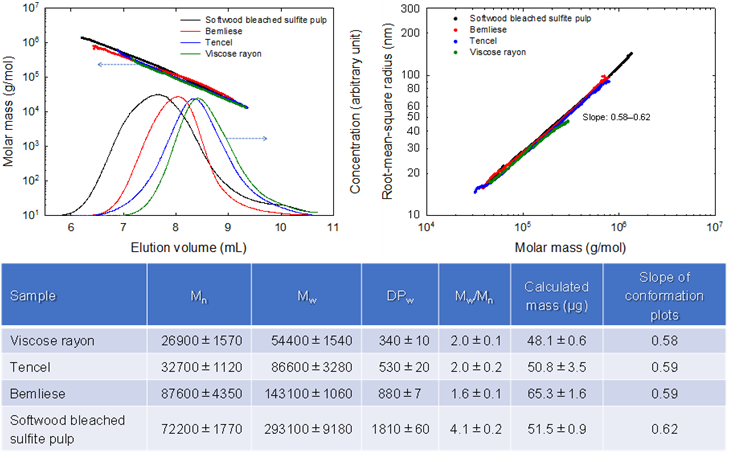
(Ono et al., Cellulose, 2023)
Changes in water-vapor-adsorption isotherms of pulp fibers and sheets during paper recycling, including drying of wet webs, and disintegration and sonication of dried sheets in water
A never-dried (ND) fines-free softwood bleached kraft pulp was converted to air-dried and thermally dried handsheets, which were then disintegrated or sonicated in water under various conditions. These disintegrated or sonicated pulps were converted to handsheets and used to obtain fundamental data on paper recycling. The water-vapor-adsorption isotherms of the pulp and sheet samples after super-critical-point drying showed clear differences between the water volumes adsorbed by the ND pulp, once-dried pulp, and dried sheets at the same relative humidities above 50%. These differences are caused by hornification of the pulp and sheet samples during drying and recycling. Air and thermal drying of wet webs decreased the adsorbed-water-vapor volume by 7%–9% and 14%–18%, respectively, relative to that adsorbed by the original ND pulp. We hypothesize that the decrease in water-vapor-adsorption volume from that of the original ND pulp at relative humidities >50% reflects the degree of irreversible formation of hydroxyl groups in the originally hydrophilic hemicelluloses and crystalline cellulose microfibril surfaces in the pulp and sheet samples during drying and paper recycling. The water-vapor-adsorption isotherms of pulp and sheet samples can be used to quantify the degree of hornification or the amount of irreversible hydrogen bonds formed during paper recycling.
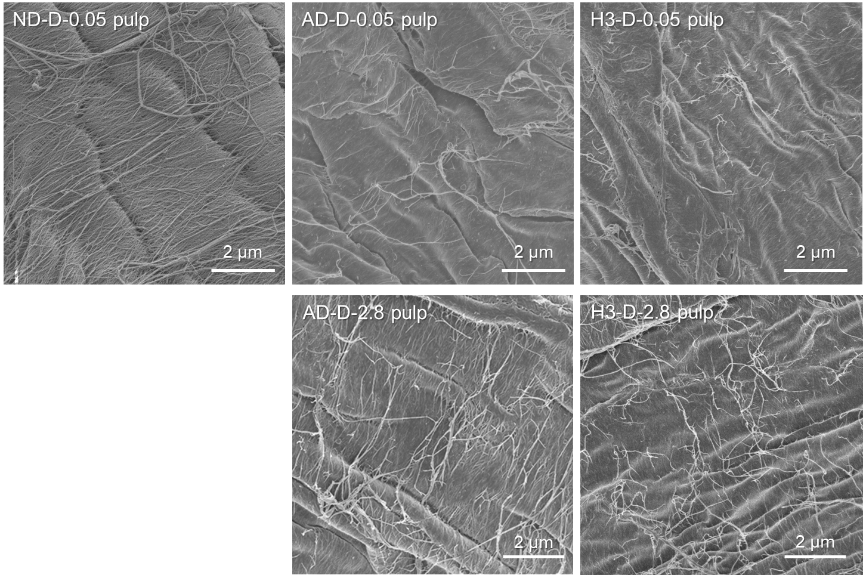
(kimura et al., Nordic Pulp & Paper Research Journal, 2023)
Changes in chemical structures and molar mass parameters of birch wood powder by ethylene diamine treatment
It is necessary to delignify wood samples and treat them with ethylene diamine (EDA) before they are dissolved in 8% (w/v) lithium chloride/N,N-dimethylacetamide (LiCl/DMAc) prior to size-exclusion chromatography (SEC) analysis. In the present study, the effects of delignifying birch wood powder 0–3 times with NaClO2 and subsequently treating it with EDA on its solubility in 8% (w/v) LiCl/DMAc and its SEC data. The neutral sugar composition of the birch powder was almost unaffected by either delignification or treatment with EDA. Treatment of the birch powder with EDA resulted in 28% solubilities in 8% (w/v) LiCl/DMAc. Approximately 11% of cellulose molecules in the birch wood powder was dissolved, and detected as a high-molar-mass (HMM) fraction in the SEC elution pattern. Each single delignification treatment increased the solubility in 8% (w/v) LiCl/DMAc to 68–74% after EDA treatment. Based on the glucose contents of the delignified samples, almost all cellulose molecules in the delignified samples were dissolved in 8% (w/v) LiCl/DMAc after EDA treatment, and detected as the HMM fractions in the SEC elution patterns. The HMM cellulose molecules in the EDA-treated birch powder had linear random-coil conformations in 1% (w/v) LiCl/DMAc. However, the SEC data suggest that there probably were some chemical linkages between the HMM cellulose molecules and lignin or NaClO2-treated lignin fragments in the HMM fractions.
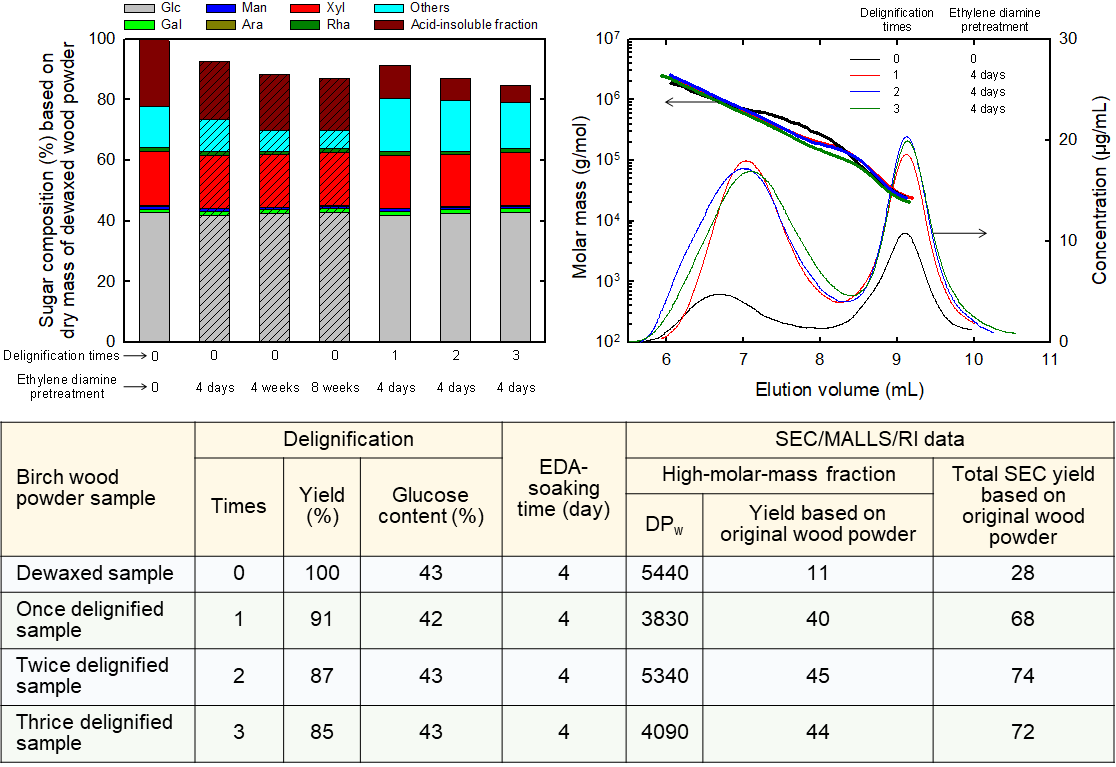
(Ono et al., Journal of Wood Chemistry and Technology, 2023)
Attachable/Detachable Sheets Containing Foam- Rubber Layers with Super Deodorant Functions
Two-layered sheets consisting of a cellulose-nanofibril-containing foam-rubber layer and a base film or non-woven sheet were developed as attachable/detachable sheets with super deodorant functions. TEMPO-oxidized cellulose nanofibrils with copper carboxylate groups (TOCNs-Cu) were composited with foam-rubber layers, which were coated on base films and sheets. These TOCNs-Cu-containing foam sheets adsorb H2S and NH3 gases well because these gas molecules are chemically or physically trapped by the foam layers. The TOCNs-Cu moieties are primarily located at the air/rubber interfaces in the foam-rubber layer. The peel and tack strengths of the TOCNs-Cu-containing foam sheets are appropriate for manual foam sheet attachment to, and detachment from, flat glass plates and plastic/ceramic boards. The H2S- and NH3-adsorption behaviors are affected by the air permeability of the base film or non-woven sheet. Transmission electron microscopy images show that TOCNs-Cu aggregates are located at the air/rubber interfaces, which results in super deodorant functions.
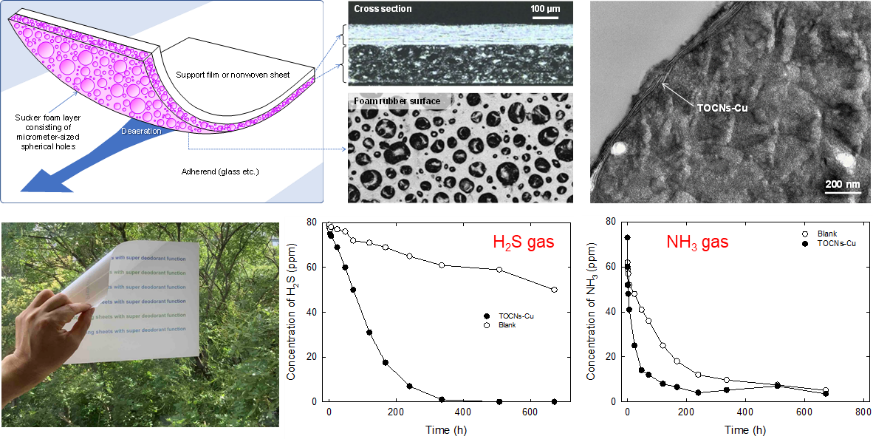
(Sone and Isogai, Industrial and Engineering Chemistry Research, 2023)
Fluoride ion adsorption isotherms, kinetics, and thermodynamics on iron(III) oxyhydroxide powders containing cellulose nanofibrils
The adsorption isotherms, kinetics, and thermodynamics of fluoride ions (F−) on FeOOH powders in water were investigated to obtain fundamental information on FeOOH powders, which are used as F− adsorbents in drinking and industrial water, and industrial wastewater. FeOOH powders were prepared as precipitates by mixing aqueous FeCl3 and NaOH solutions (1:3 mol/mol) in the presence of 2,2,6,6,-tetramethylpiperidine-1-oxyl radical (TEMPO)-oxidized cellulose nanofibrils (TOCNs), carboxymethylcellulose (CMC), or TEMPO-oxidized cellulose (TOC) fibers (without nanofibrillation), and subsequent drying and pulverizing. The FeOOH:TOCN, FeOOH:CMC, and FeOOH:TOC dry mass ratios were controlled at 87:13. The amount of F− adsorbed by the FeOOH/TOCN powder per FeOOH mass was higher than those adsorbed by FeOOH, FeOOH/CMC, or FeOOH/TOC. The F− adsorption isotherms on the FeOOH-containing powders showed higher correlation coefficients with the Langmuir model than with the Freundlich model. This indicates that F− adsorbed on FeOOH initially formed a monolayer, predominantly via physical adsorption. Pseudo-second-order kinetics fitted well to the time-dependent F− adsorption behaviors on the FeOOH-containing powders. Thermodynamic analysis of F− adsorption on the FeOOH-containing powders showed that the ΔG values were negative, which indicates that F− adsorption on the FeOOH-containing powders proceeded spontaneously in water. The negative ΔG value for FeOOH/TOCN was higher than those for FeOOH, FeOOH/CMC, and FeOOH/TOC at the same temperature. This shows that the FeOOH/TOCN powder can be used as an excellent and efficient F− adsorbent in water.
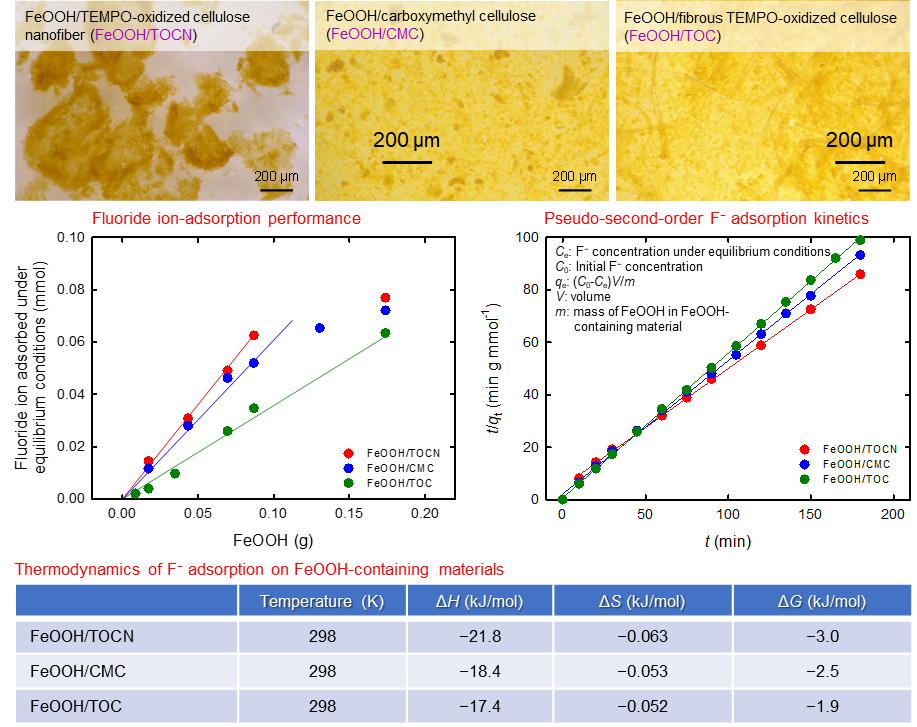
(Umehara et al., Environmental Science and Pollution Research, 2023)
Mechanically robust, flame-retardant phosphorylated cellulose films with tunable optical properties for light management in LEDs
Biodegradable cellulose films with excellent mechanical, optical, and functional properties have attracted considerable attention as promising alternatives to plastics for photoelectronic devices. In this work, mechanically ductile, flame-retardant cellulose films with tunable optical properties were prepared by simple mechanical disintegration of phosphorylated cellulose (PhC) fibers, vacuum filtration of as-prepared dispersions, and subsequent pressing of the wet PhC films to prepare dried films. When mechanical disintegration conditions were optimized, the resultant cellulose PhC films exhibited an average density, tensile strength, Young’s modulus, tensile toughness, and folding resistance of 1.4 g/cm3, 150 MPa, 8.5 GPa, 8.2 MJ/m3, and 4580 times, respectively. The PhC film hazes were widely controllable from 9% to 91%, while they maintained high light transmittances (>90%) at a 550-nm wavelength. The PhC films were used for light management of light-emitting diodes by controlling mechanical fibrillation conditions of the PhC fiber/water slurry, showing that the films effectively improved the luminescence uniformity of the devices.
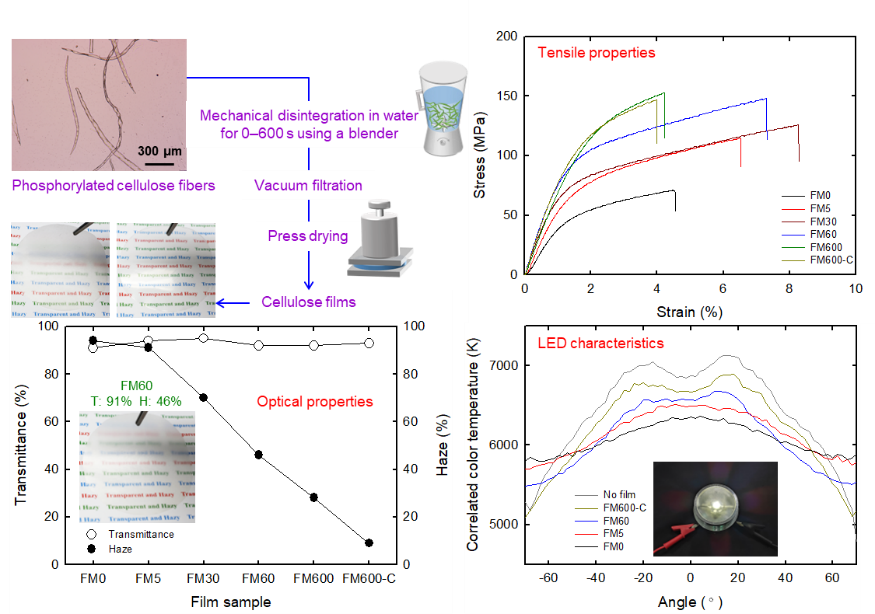
(Hou et al., Carbohydrate Polymers, 2022)
Characterization of solid-state structures, molar masses, and microfibril structures of cellulose in never-dried cotton fibers and ramie bast fibers
Commercial cellulose is dried and/or hydrothermally treated under various conditions during isolation and purification. These processes may influence the structures and properties of the resulting cellulose products. In the present study, never-dried ramie and cotton cellulose samples were prepared from ramie bast and cotton fibers by delignification and KOH treatment. The neutral sugar compositions, X-ray diffraction (XRD) patterns, solid-state carbon 13 nuclear magnetic resonance (13C-NMR) spectra, and molar masses of the cellulose samples were determined to elucidate their chemical and crystal structures. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) images of TEMPO-oxidized cellulose nanofibrils prepared from the cellulose samples were examined to determine their microfibril structures and morphologies (TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxyl radical). The highest post-treatment glucose contents of the ramie and cotton cellulose samples were 93% and 98%, respectively. Cellulose I crystal widths in the ramie and cotton cellulose samples, calculated from their XRD patterns, were 3.5–3.8 nm and 4.6–5.1 nm, respectively. The ramie and cotton cellulose samples produced by pre-treating the never-dried samples under suitable conditions had higher molar masses than commercial products. The fibril widths determined from the AFM height images of the TEMPO-oxidized cellulose nanofibrils prepared from the never-dried ramie and cotton cellulose samples were 2.8 ± 0.7 nm and 3.1 ± 0.8 nm, respectively. These values were smaller than those of commercial ramie and cotton cellulose fibers. Therefore, the large cellulose I crystal widths found in commercial ramie and cotton cellulose fibers may result from the drying conditions of those fibers during production.
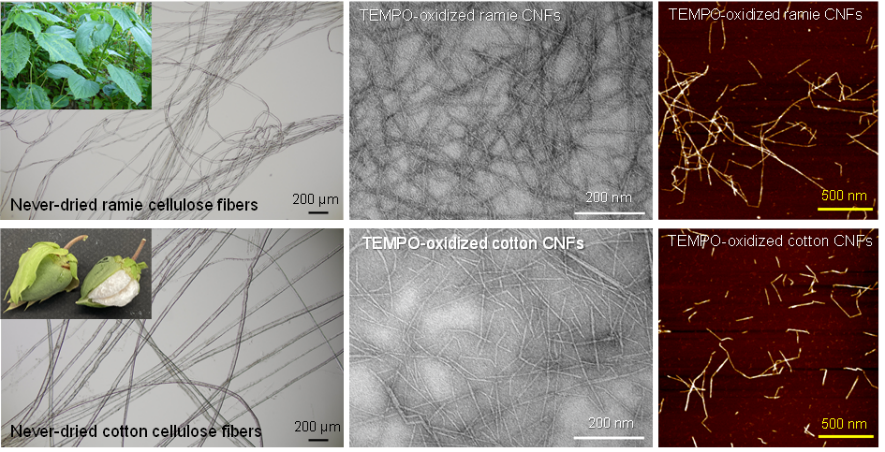
(Ono et al., Cellulose, 2022)
Effects of Surface Chemistry and Counterion Selection on the Thermal Behavior of Carboxylated Cellulose Nanocrystals
Researchers have sought to improve the thermal performance of cellulose nanocrystals (CNCs) via new production routes, often by imparting carboxylate groups on CNC surfaces. The CNC properties responsible for increased thermal stability, however, are not well understood. This study investigated carboxylated CNCs with varying physicochemical properties and benchmarked their thermal performance with two counterions (H+ and Na+) against typical sulfated CNCs. Carboxylated CNCs were more thermally stable in acid form than sodium form (the opposite of sulfated CNCs), highlighting that CNCs with different surface chemistries cannot be compared with the same counterion when making claims about thermal behavior. Thermogravimetric analysis and solid-state NMR spectroscopy were used to evaluate five types of CNCs. Overall, sulfate group content affected CNC thermal stability more than carboxylate content – sulfated CNCs showed both the highest and lowest onsets of thermal degradation, in sodium and acid form, respectively. Carboxylated CNCs displayed a variety of “intermediate” thermal behaviors: CNCs with higher carboxylate contents and larger specific surface areas had lower degradation temperatures and tended towards one main pyrolysis step (instead of two) as surface area increased. The catalytic effect of sodium in highly charged TEMPO-oxidized carboxylated CNCs, and the formation of sodium carbonate in moderately charged, esterified CNCs, led to more structural degradation of cellulose in sodium form than acid form. Due to the generated sodium carbonate, decarboxylation reactions upon heating decreased for esterified CNCs in sodium form, but increased in acid form. This new understanding will allow optimized performance of CNC-based materials at high temperatures and may enable the development of bionanomaterials with targeted thermal properties.
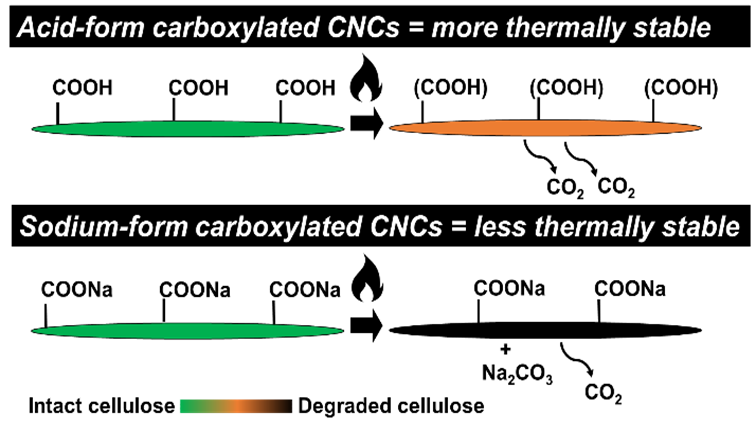
(Vanderfleet et al., Chemistry of Materials, 2022)
Iron (III) oxyhydroxide powders with TEMPO-oxidized cellulose nanofibrils: effective adsorbents for removal of fluoride ion in water
Fluoride ions are toxic, and F− in groundwater for drinking should be sufficiently reduced to be less than the regulated concentration. In this study, cellulose nanofibril-containing iron oxyhydroxide (FeOOH) powders were synthesized, and their F− adsorption behaviors were studied under various conditions. 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose nanofibrils (CNFs) were added to 0.1 M FeCl3, and 1 M NaOH was added to the FeCl3/TEMPO–CNF mixture to prepare FeOOH precipitates at various FeOOH/TEMPO–CNF mass ratios. When TEMPO–CNFs were present in the FeCl3 solution, the FeOOH/TEMPO–CNF precipitates were quantitatively obtained by straightforward filtration with filter paper; enabled by the larger size (>300 µm) of the FeOOH/TEMPO–CNF precipitates than the FeOOH precipitates (<100 µm) prepared without TEMPO–CNFs. The oven-dried FeOOH/TEMPO–CNF powder (87/13 by mass) showed the highest F− adsorption ratio in water compared with the synthesized FeOOH/TEMPO–CNF powders with FeOOH/TEMPO–CNF mass ratios of 100/0, 79/21, and 73/27. The F− adsorption ratios of the FeOOH/TEMPO–CNF powders were stable over the pH range 4‒10. Scanning electron microscopy and energy-dispersive spectroscopy analyses of the powder surfaces before and after F− adsorption indicate that the F− in water adsorbed onto the FeOOH/TEMPO–CNF powder (87/13 by mass) and partially replaced the Cl− that was originally present in the powder. Thus, the FeOOH/TEMPO–CNF powder prepared in this study can be used as efficient, cost-effective F− adsorbents for drinking water and industrially polluted wastewater.
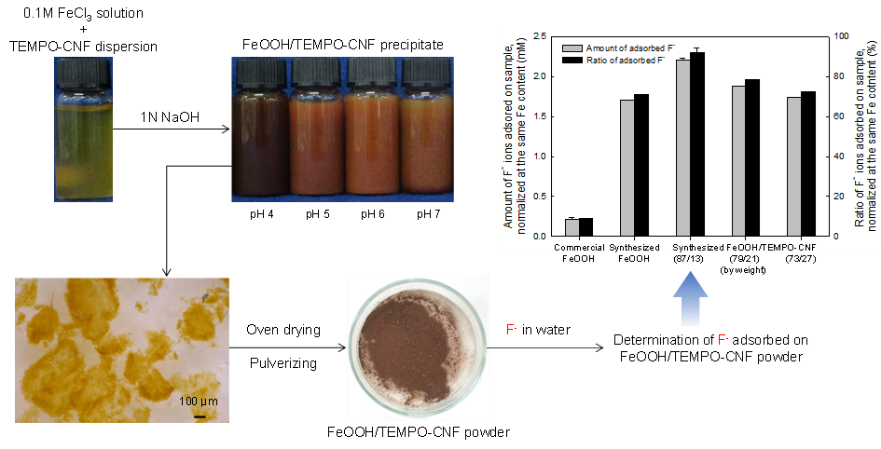
(Umehara et al., Cellulose, 2022)
Real-time observation of microcrack growth in nanocellulose/rubber composite sheets during tensile deformation process
Rubber/nanocellulose composite sheets improve tensile properties compared with neat rubber sheets, when nanocellulose materials are suitably compounded with rubber matrices. First, two oven-dried nanocellulose materials containing different additive components were prepared from nanocellulose/water dispersions, and the oven-dried nanocellulose-containing materials were compounded with rubber sheets under high shear forces by using a two-roll mill. The tensile moduli and strength of the rubber/nanocellulose composite sheets were clearly better than those of the rubber sheet prepared without nanocellulose, while the elongations at break were similar. Thin films were cut from the rubber/nanocellulose composite sheets, and their real-time tensile deformation processes were observed by transmission electron microscopy (TEM) to understand the time-dependent patterns of crack propagation, void formations, and fusion between cracks and voids in the composites during tensile deformation. TEM observations showed that the composite sheets consisted of individual rubber/nanocellulose clusters. Voids initially formed inside the rubber/nanocellulose clusters, propagated to form cracks, fused with other voids or secondary cracks. There were slight differences between the crack-propagation patterns of the two rubber composites, probably because of differences in the morphologies, sizes, distributions, and structures of the rubber/nanocellulose clusters, and surface chemical structures of individual nanocellulose elements between the composites.
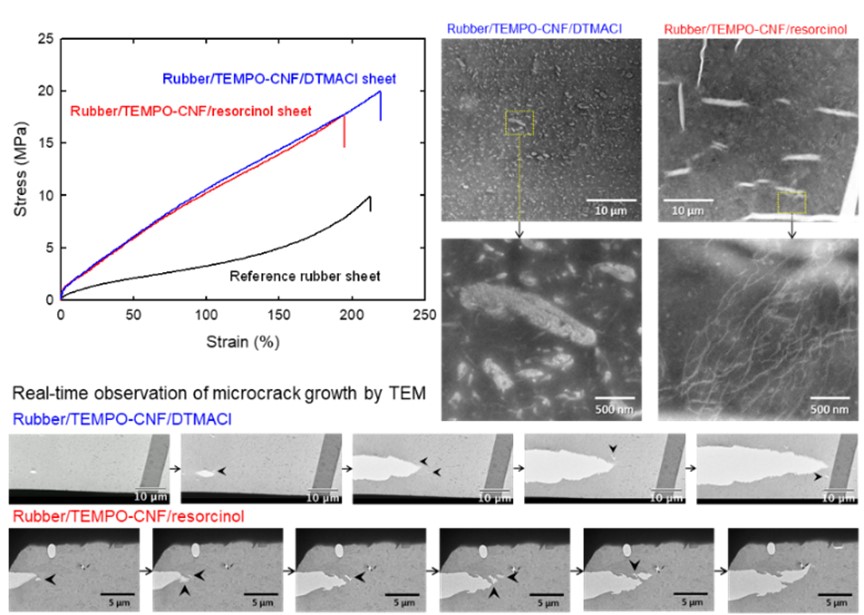
(Jinnai et al., Polymer Composites, 2022)
A systematic study for the structures and properties of phosphorylated pulp fibers prepared under various conditions
A bleached softwood kraft pulp was phosphorylated with (NH4)2HPO4 and urea at 150 °C for 0‒40 min, and the structures and properties of the resulting phosphorylated pulps were systematically investigated for the first time in terms of reaction time and the amount of (NH4)2HPO4 added. The phosphorous and weak acid group contents, and the weight recovery ratio increased with increasing reaction time, and were 1.9 and 4.5 mmol/g, and 114%, respectively, when the reaction time was 20 min. Therefore, numerous phosphate ester and weak acid groups were introduced into the pulp, maintaining its fibrous morphology and cellulose I crystal structure. It was found that almost all the ammonium phosphate groups in the phosphorylated pulp behaved as weak acids. The solid-state carbon-13 nuclear magnetic resonance (13C-NMR) spectrum of the phosphorylated pulp showed that neither carboxy nor carbamate groups were formed in the phosphorylated pulp; only phosphate ester groups were introduced into the pulp under the conditions used in the present study. The X-ray photoelectron spectra of the phosphorylated pulp surfaces suggested that ammonium phosphate groups were introduced into the pulp by phosphorylation. A longer reaction time or a greater amount of (NH4)2HPO4 added during phosphorylation resulted in lower water swelling behavior, indicating that some intrafiber and/or interfiber crosslinking occurred in the pulp under these conditions. The results obtained in the present study show that phosphorylated pulp fibers are suitable for the preparation of new functional cellulosic sheets and materials, in which large amounts of weak acid groups can be used as scaffolds for diverse ion-exchange sites.
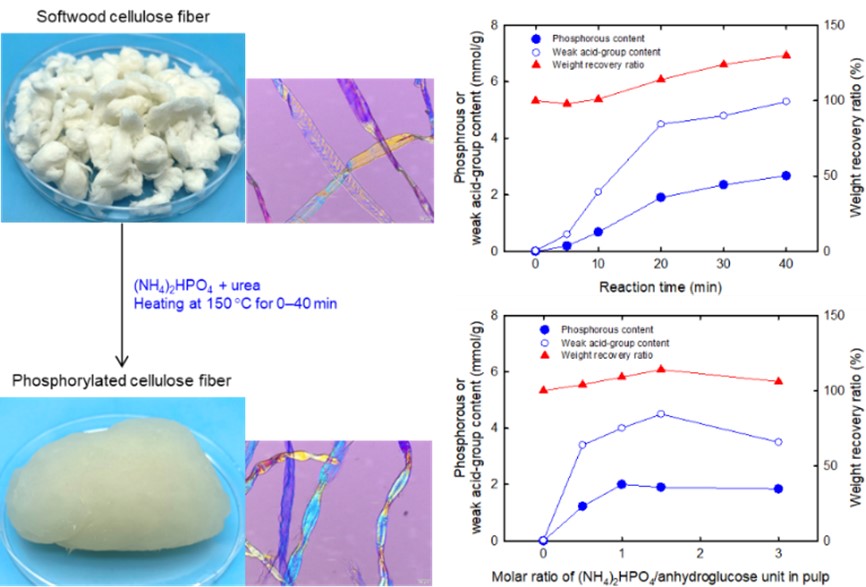
(Hou et al, Cellulose, 2022)
Cellulose nanofibril/polypropylene composites prepared under elastic kneading conditions
An aqueous dispersion of 2,2,6,6,-tetramethylpiperidine-1-oxyl radical (TEMPO)-oxidized cellulose nanofibrils (TEMPO-CNFs) was mixed with diethylene glycol (DEG) and dodecyltrimethylammonium chloride (DTMACl) with or without silane coupling agents. The mixture was heated at 40 °C for 1 d to prepare an oven-dried TEMPO-CNF/DEG/DTMACl, which was added to maleic anhydride-modified polypropylene (MA-PP) and kneaded at 165‒175 °C with high shear forces to prepare TEMPO-CNF/MA-PP master batches. Various amounts of TEMPO-CNF/MA-PP master batch pieces were mixed with PP to prepare TEMPO-CNF/MA-PP/PP composite sheets. The yield stress and storage modulus at 25 °C of the composite sheets increased almost linearly with an increase in TEMPO-CNF content. However, the elongation at break decreased clearly with TEMPO-CNF content because of partial formation of TEMPO-CNF aggregates in the composites. The presence of TEMPO-CNFs restricted flow behavior of the MA-PP/PP components above 160 °C, although the crystallinities and melting behavior of MA-PP/PP in the composite sheets at ~160 °C were unchanged. The apparent aspect ratios of TEMPO-CNF components in the composite sheets were 5‒13 by partial aggregation of TEMPO-CNFs in the PP matrix, although the aspect ratio of the original TEMPO-CNFs dispersed in water was ~183. The aggregation behavior of TEMPO-CNFs in the PP matrix may have resulted in brittle tensile properties of the composite sheets. The TEMPO-CNF-containing PP sheets have better printability and adhesion performance between sheets using glues. These results indicate that the oven-dried TEMPO-CNFs can be used as fillers for improvement of mechanical, thermal, and printing properties of recycled and low-quality PP and for quantitative expansion of recycled PP.
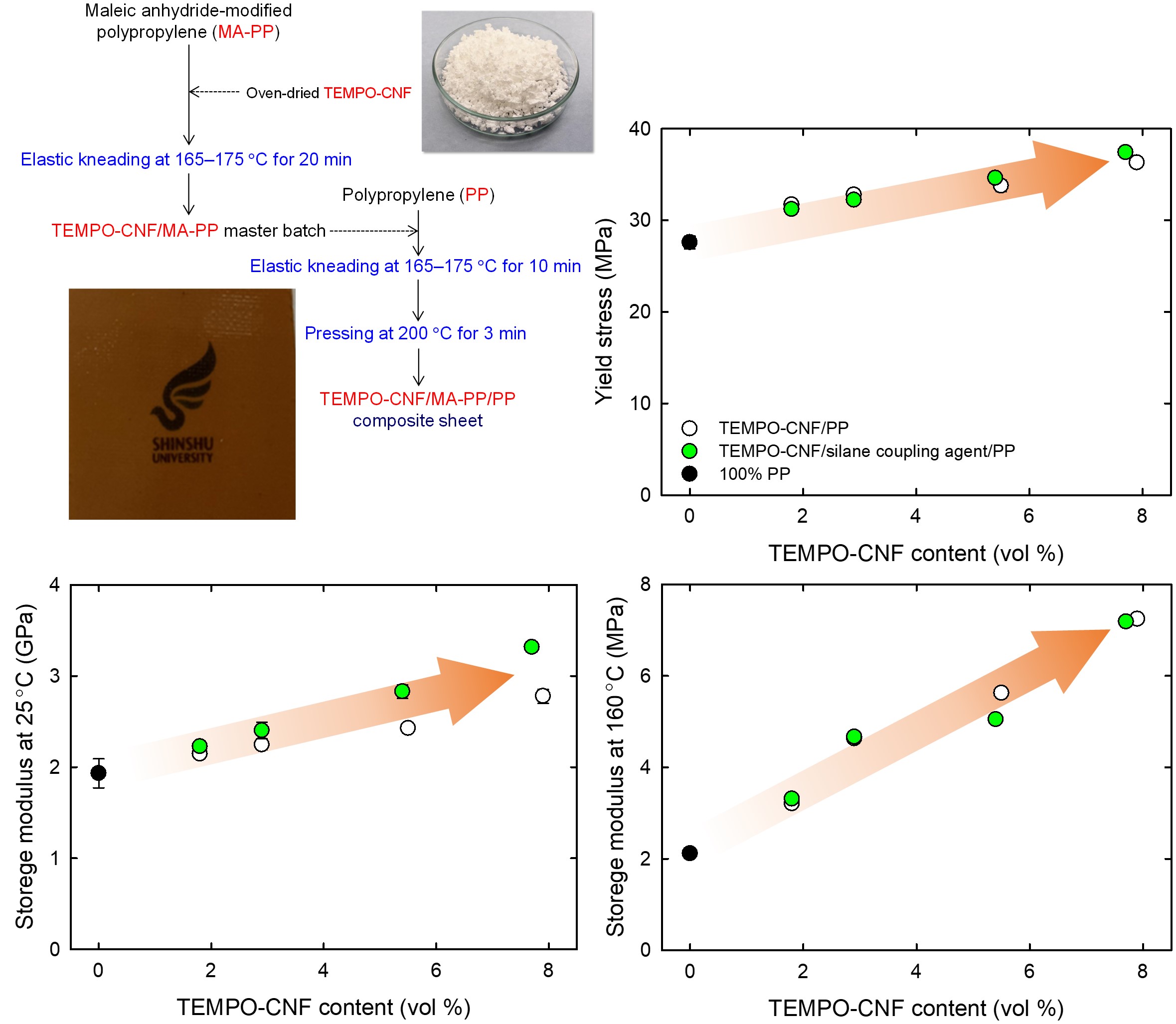
(Noguchi et al., Cellulose, 2022)
Structures, molar mass distributions, and morphologies of TEMPO-oxidized bacterial cellulose fibrils
2,2,6,6-Tetramethylpiperidine-1-oxyl radical (TEMPO)-mediated oxidation has been applied to bacterial cellulose (BC). The TEMPO-oxidized BC (TO-BC) gel particles were subjected to ultrasonication in water to prepare mechanically fibrillated TO-BC (TO-BC-U) samples. The carboxyl contents of the TO-BC samples were 1.5‒1.6 mmol/g. X-ray diffraction patterns and solid-state 13C-nuclear magnetic resonance (NMR) spectra of the BC, TO-BC, and TO-BC-U samples showed that cellulose Iα was the dominant crystalline structure. The crystallinities of the samples calculated from the carbon signal areas in the NMR spectra were approximately the same between the BC and TO-BC samples, showing that TEMPO-mediated oxidation selectively occurred on the crystalline BC fibril surfaces. However, the crystallinities of the TO-BC-U samples were lower than those of the BC and TO-BC samples, indicating that ultrasonication of the TO-BC samples in water caused partial decreases in crystallinity. The TO-BC-U samples contained both single fibrils and fibril bundles; completely individualized TO-BC-U fibrils with homogeneous widths was not obtained. The average widths of the single TO-BC-U fibrils were ~3 nm, which are close to those of TO-cellulose nanofibrils prepared from wood-cellulose samples. Thus, the crystalline BC fibrils with widths of ~3 nm were the smallest crystalline elements. The lengths of the TO-BC samples were greater than 2‒3 µm, whereas the weight-average cellulose chain lengths of the cellulose/TEMPO-oxidized cellulose molecules in TO-BC-U samples were <800 nm. Hence, each TO-BC-U fibril consisted of multiple cellulose and oxidized cellulose molecules, which were packed along the longitudinal direction.
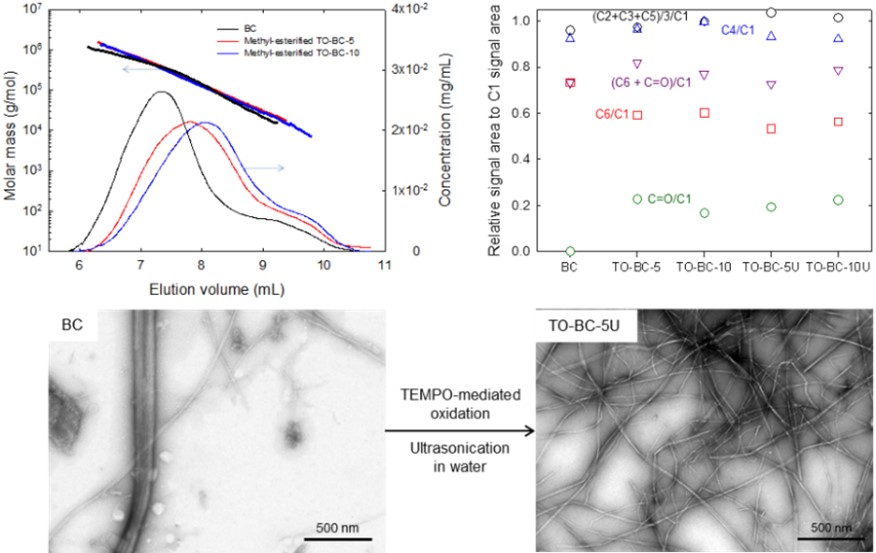
(Ono et al., Cellulose, 2022)
Changes in neutral sugar composition, molar mass and molar mass distribution, and solid-state structures of birch and Douglas fir by repeated sodium chlorite delignification
NaClO2-treated residues or holocellulose samples were prepared from birch and Douglas fir wood powders by repeated cycles of delignification. These delignified samples were characterized from their weight recovery ratios, neutral sugar compositions, size-exclusion chromatograms (SECs) using 1% (w/v) lithium chloride/N,N-dimethylacetamide as eluent, and solid-state 13C nuclear magnetic resonance (NMR) spectra. Neutral sugar composition analysis revealed that the weight ratios of cellulose and xylan fractions were almost constant irrespective of the number of repeated delignification cycles for up to seven and eight cycles for birch and Douglas fir, respectively. The contents of acetyl ester groups were constant between the delignified samples. The glucomannan molecules present in Douglas fir were partly removed from the delignified residues as the number of delignification cycles increased. The SEC analysis showed that the high-molar-mass (HMM) cellulose fractions of birch samples decreased slightly in molar mass with increased number of delignification cycles. The molar mass values in the HMM fractions of Douglas fir samples were much higher than those of the birch samples because of the presence of branched structures with glucomannan, and were mostly unchanged as the number of delignification cycles increased. Solid-state 13C NMR analysis of the NaClO2-treated residues and their acid-insoluble fractions showed that carboxy groups were formed in the residual lignin fractions by the oxidative delignification treatment with NaClO2. Thus, neutral sugar composition, SEC analysis, and solid-state 13C NMR analysis can be used for comprehensive and manifold characterization of NaClO2-treated residues or holocellulose samples prepared from various plant samples.
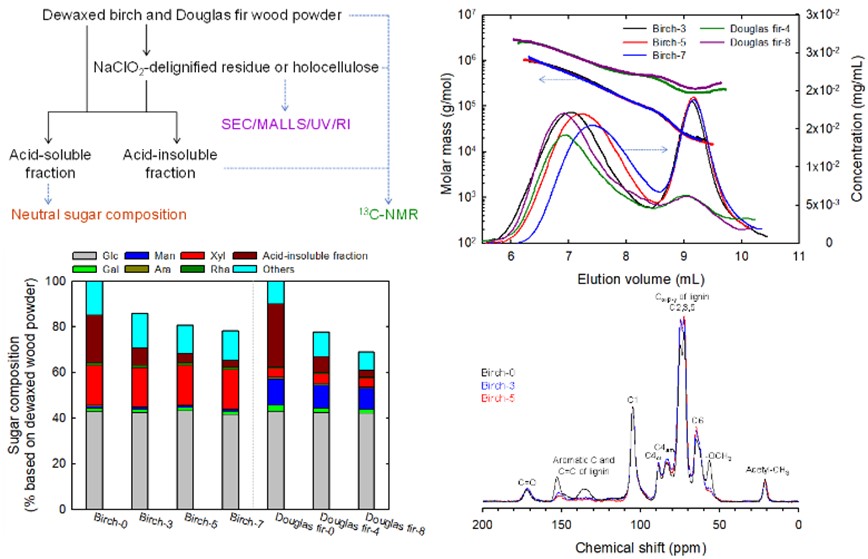
(Ono et al., Cellulose, 2022)
Silver-nanoparticle-containing handsheets for antimicrobial applications
Development of self-sanitizing cellulose and cellulose paper-based products will increase human safety and hygiene. In the present work, a softwood bleached kraft pulp (SBKP) was oxidized by 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO)-mediated oxidation in water at pH 10 at two NaClO addition levels (3 and 5 mmol g−1 based on the dry weight of SBKP). The fibrous TEMPO-oxidized SBKPs (TO-SBKPs) were subsequently incorporated with silver nanoparticles (AgNPs) by soaking in aqueous silver nitrate (AgNO3) solution and subsequent thermal reduction. The C=O absorption band in FTIR spectra of AgNP-containing TO-SBKPs increased with increasing Ag content, showing that the C2/C3 hydroxy groups in TO-SBKPs were oxidized to ketones by reduction of Ag+ ions to AgNPs during heating at 100 °C for 1 h. Scanning electron microscopy images showed that the AgNPs were almost homogenously distributed on the surface of each TO-SBKP fiber with an average diameter of 32–40 nm regardless of different Ag contents. Handsheets were prepared from SBKP and the AgNP-containing TO-SBKP at various weight ratios. The handsheets showed sufficient antimicrobial activities against a Gram-negative Escherichia coli strain and a Gram-positive Staphylococcus aureus strain. The tensile strength of the handsheets was significantly improved by mixing the AgNP-containing TO-SBKP with SBKP. The 20% TO-SBKP/Ag-containing SBKP sheets were optimal in terms of efficient antimicrobial activities and good mechanical properties. Thus, the AgNP-containing TO-SBKP sheets have potential for use as antimicrobial paper and related packaging materials produced using the conventional papermaking process.
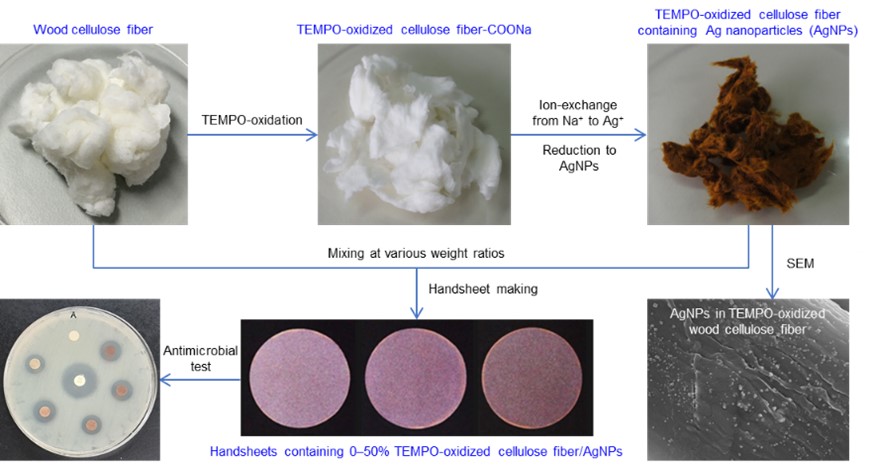
(Puangshin et al., Cellulose, 2022)
Copper-coordinated cellulose ion conductors for solid-state batteries
Although solid-state lithium (Li)-metal batteries promise both high energy density and safety, existing solid ion conductors fail to satisfy the rigorous requirements of battery operations. Inorganic ion conductors allow fast ion transport, but their rigid and brittle nature prevents good interfacial contact with electrodes. Conversely, polymer ion conductors that are Li-metal-stable usually provide better interfacial compatibility and mechanical tolerance, but typically suffer from inferior ionic conductivity owing to the coupling of the ion transport with the motion of the polymer chains. Here we report a general strategy for achieving high-performance solid polymer ion conductors by engineering of molecular channels. Through the coordination of copper ions (Cu2+) with one-dimensional cellulose nanofibrils, we show that the opening of molecular channels within the normally ion-insulating cellulose enables rapid transport of Li+ ions along the polymer chains. In addition to high Li+ conductivity (1.5 × 10−3 siemens per centimetre at room temperature along the molecular chain direction), the Cu2+-coordinated cellulose ion conductor also exhibits a high transference number (0.78, compared with 0.2–0.5 in other polymers) and a wide window of electrochemical stability (0–4.5 volts) that can accommodate both the Li-metal anode and high-voltage cathodes. This one-dimensional ion conductor also allows ion percolation in thick LiFePO4 solid-state cathodes for application in batteries with a high energy density. Furthermore, we have verified the universality of this molecular-channel engineering approach with other polymers and cations, achieving similarly high conductivities, with implications that could go beyond safe, high-performance solid-state batteries.
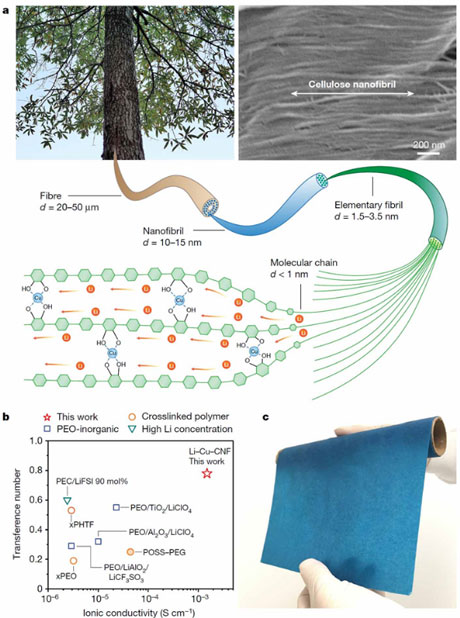
(Yang et al., Nature, 2021)
TEMPO-catalyzed oxidation of polysaccharides
2,2,6,6-Tetramethylpiperidine-1-oxyl radical (TEMPO)-catalyzed oxidation enables efficient and position-selective conversion of primary hydroxy groups in water-soluble and water-insoluble polysaccharides to sodium carboxylate groups under mild conditions in water. TEMPO/NaBr/NaClO in water at pH 10 is an advantageous system in terms of the degrees of oxidation and reaction rates. TEMPO and NaBr behave as catalysts, and NaClO acts as the primary oxidant. However, oxidative depolymerization that is caused by the presence of NaBr and NaClO and the occurrence of side reactions on the polysaccharide molecules are unavoidable during oxidation. An alternative system is 4-acetamido-TEMPO/NaClO/NaClO2 in pH 5–7 buffer at 35–60 °C for 1–3 d, in which catalytic amounts of TEMPO and NaClO are used with NaClO2 as the primary oxidant. This oxidation system significantly inhibits depolymerization and yields oxidized products that contain no aldehydes. Various new water-soluble TEMPO-oxidized polysaccharides that contain significant amounts of sodium carboxylate groups have been prepared by TEMPO-catalyzed oxidation, and they have unique properties and functionalities. When crystalline native cellulose and chitin are oxidized by the TEMPO/NaBr/NaClO system under suitable conditions, the obtained water-insoluble oxidized products can be converted to various characteristic nanomaterials by mechanical disintegration in water, depending on the oxidation and disintegration conditions.
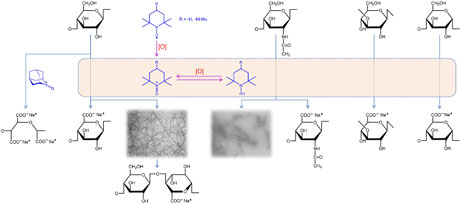
(Isogai, Polymer Journal, 2021)
Cellulose-nanofiber-reinforced rubber composites with resorcinol resin prepared by elastic kneading
A resorcinol resin/water dispersion and a rubber latex are added to 1% 2,2,6,6,-tetramethylpyperidine-1-oxyl (TEMPO)-oxidized cellulose nanofibers (TEMPO-CNFs) dispersed in water, followed by oven drying at 40 °C for 20 h to prepare a dried TEMPO-CNF/resorcinol resin/latex rubber (DTRL) mixture with a weight ratio of 1/0.5/3. DTRL is then added to a nitrile-butadiene rubber (NBR) or a carboxy group-containing NBR (X-NBR) sheet, and the mixture is kneaded by a two-roll mill at 20–30 °C with high shear forces. The tensile strength and Young’s modulus of the crosslinked DTRL/rubber composite sheets remarkably increased from 10 and 12 MPa, respectively, for the reference sheet to 24 and 82 MPa, respectively, for the DTRL/rubber composite sheets containing ≈10 vol% TEMPO-CNFs. Scanning electron microscopy revealed that no TEMPO-CNF agglomerates are present in the DTRL/rubber composite sheets. The tensile properties of the composite sheets are the best when a X-NBR sheet and NBR latex are used as the matrix rubber and latex in DTRL preparation, respectively. When water-extracted DTRL (WDTRL, mass recovery ratio ≈94%) is used in place of DTRL, the WDTRL/rubber composite sheets show sufficient water resistance, while the tensile properties are almost the same as those of the DTRL/rubber composite sheets.
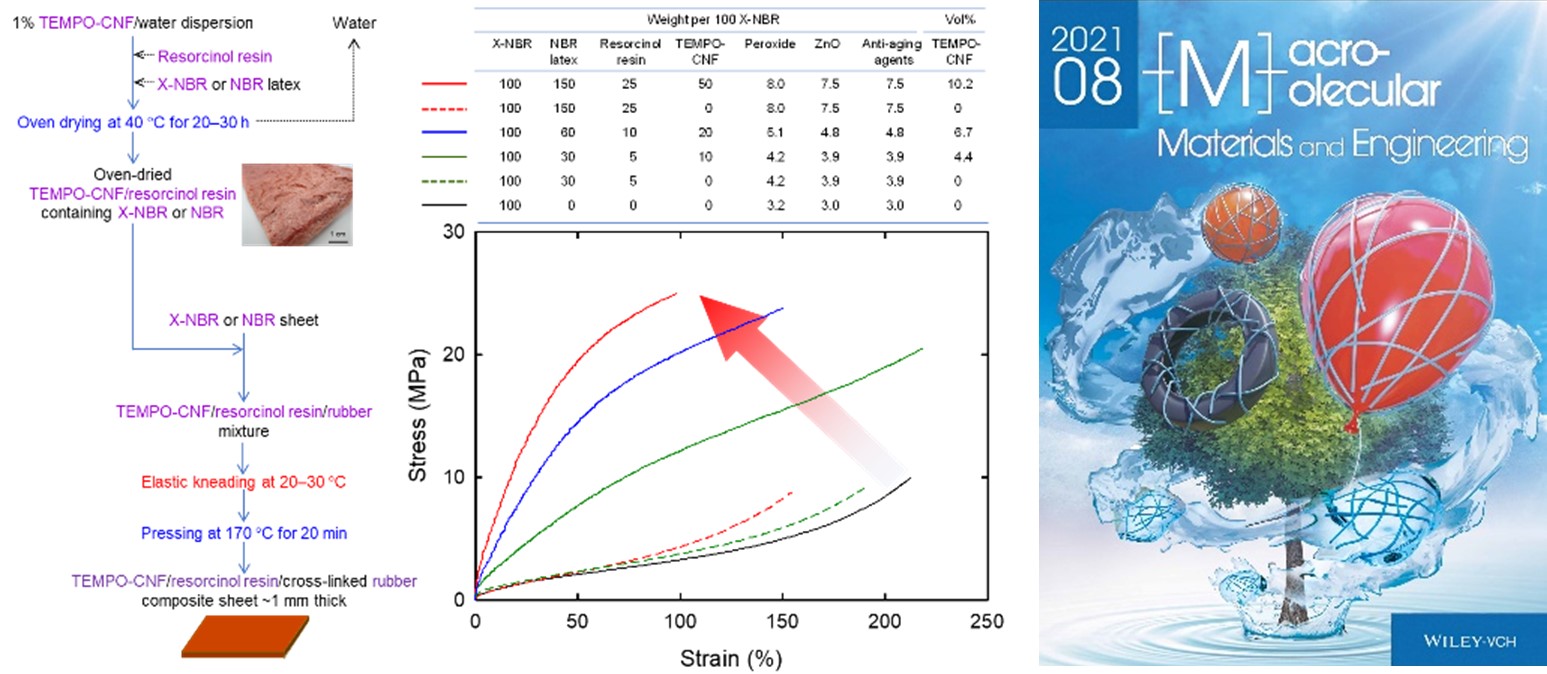
(Noguchi et al., Composite Science and Technology, 2021)
TEMPO/NaBr/NaClO and NaBr/NaClO oxidations of cotton linters and ramie cellulose samples
Dried cotton linters and ramie cellulose samples were oxidized with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)/NaBr/NaClO and NaBr/NaClO (i.e., TEMPO-free) in water at pH 10. The carboxy contents, degrees of polymerization (DPs), X-ray diffraction patterns, and solid-state 13C NMR spectra were measured or obtained for the oxidized products with and without subsequent NaBH4 reduction. Cellulose nanofibrils were prepared from the oxidized cellulose samples by sonication in water and observed by atomic force microscopy and transmission electron microcopy. Because the cellulose molecules were depolymerized with NaBr/NaClO, the depolymerization behavior of the cellulose samples with TEMPO/NaBr/NaClO can be mainly explained by depolymerization with NaBr/NaClO (i.e., not TEMPO-related compounds or reactions). However, because C6-aldehydes formed in the disordered regions periodically present along the longitudinal direction of each cellulose microfibril, the viscosity-average DP values of the TEMPO/NaBr/NaClO-oxidized cellulose samples decreased to 200–300, while those with subsequent NaBH4 reduction exhibited much higher DP values. The nanofibrils prepared from the TEMPO/NaBr/NaClO-oxidized cellulose samples had smallest fibril heights or widths of 5–6 nm. However, significant amounts of unfibrillated bundles with heights of 10–40 mm were present in the nanofibril/water dispersions. The high carboxy contents of the TEMPO/NaBr/NaClO-oxidized cellulose samples (1.62–1.63 mmol/g) indicated that significant amounts of carboxy groups were likely present in the disordered regions, probably forming tail-like polyglucuronate chains. Solid-state 13C NMR analysis revealed that some of the glucosyl units originally with the tg C6–OH conformation were transformed to other conformations by TEMPO/NaBr/NaClO oxidation, while the crystalline C4 signal areas remained constant.
(Ono et al., Cellulose, 2021)
Nanocellulose-containing cellulose ether composite films prepared from aqueous mixtures by casting and drying method
A TEMPO-oxidized cellulose nanofibril (TEMPO-CNF)/water dispersion was mixed with an aqueous solution of hydroxypropyl cellulose (HPC), hydroxyethyl cellulose (HEC), methyl cellulose (MC), or carboxymethyl cellulose sodium salt (CMC). The mixtures were converted into TEMPO-CNF/cellulose ether composite films containing 0–5% TEMPOCNFs by casting and drying of the aqueous mixtures. All the composite films had high light transparency. However, the tensile properties of the composite films of a given TEMPO-CNF content differed for the HPC and HEC, MC, and CMC matrices. The nanoreinforcing effect owing to TEMPO-CNFs was evident in the TEMPO-CNF/HPC composite films, which became strong but brittle. In contrast, the TEMPOCNF/HEC composite films did not exhibit this nanoreinforcing effect. Transmission electron microscopy observation of the film cross-sections revealed that the TEMPO-CNF elements were homogeneously distributed in the 5% TEMPO-CNF/HPC composite film. In contrast, the TEMPO-CNF elements were densely present on the top and bottom surfaces of the 5% TEMPO-CNF/HEC composite film; the TEMPOCNFs were heterogeneously distributed in the HEC matrix. The low gelation or aggregation concentrations of TEMPO-CNFs in water may have resulted in the different distribution states of TEMPO-CNFs, depending on the cellulose ether matrix used, and hence the different tensile behaviors.
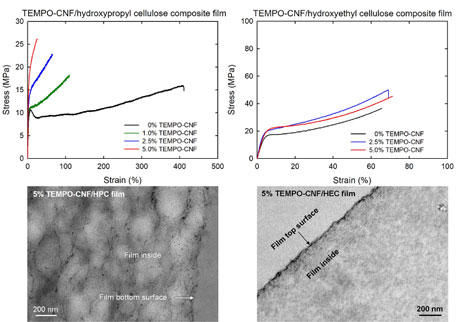
(Okahashi et al., Cellulose, 2021)
Emerging nanocellulose technologies: recent developments
Nanocelluloses have unique morphologies, characteristics, and surface nanostructures, and are prepared from abundant and renewable plant biomass resources. Therefore, expansion of the use of CO2-accumulating nanocelluloses is expected to partly contribute to the establishment of a sustainable society and help overcome current global environmental issues. Nanocelluloses can be categorized into cellulose nanonetworks, cellulose nanofibrils, and cellulose nanocrystals, depending on their morphologies. All of these materials are first obtained as aqueous dispersions. In particular, cellulose nanofibrils have homogeneous ≈3 nm widths and average lengths of >500 nm, and significant amounts of charged groups are present on their surfaces. Such charged groups are formed by carboxymethylation, C6-carboxylation, phosphorylation, phosphite esterification, xanthation, sulfate esterification, and C2/C3 dicarboxylation during the pretreatment of plant cellulose fibers before their conversion into cellulose nanofibrils via mechanical disintegration in water. These surface-charged groups in nanocelluloses can be stoichiometrically counterion-exchanged into diverse metal and alkylammonium ions, resulting in surface-modified nanocelluloses with various new functions including hydrophobic, water-resistant, catalytic, superdeodorant, and gas-separation properties. However, many fundamental and application-related issues facing nanocelluloses must first be overcome to enable their further expansion.
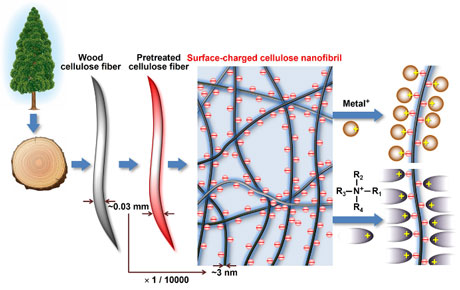
(Isogai, Advanced Materials, 2021)
Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: a review
Cellulose is the most earth-abundant natural polymer resource, which with combined eco-friendly and extraordinary sustainable properties such as renewability, biodegradability, low cost and excellent biocompatibility has been widely used by humans for thousands of years. In the past few years, many novel cellulosic materials and their unique applications have been developed including the recent research focus on energy harvesting. The high crystallization and plentiful polar hydroxyl groups endow cellulose with a large number of dipoles and strong electron donating capacity, resulting in a promising potential of piezoelectric and triboelectric effects. However, there is no review about cellulose-based nanogenerators until now. In this paper, the most recent developments of designing, modification, processing and integration of cellulose-based piezoelectric nanogenerators (PENGs), triboelectric nanogenerators (TENGs) and hybrid piezo/triboelectric nanogenerators (PTENGs) for energy harvesting and other applications are reviewed in detail. For cellulose-based PENGs, representative basic piezoelectric cellulose and recent research on PENG devices are discussed. For cellulose-based TENGs, several effective strategies including rough modification of contact surface, addition of electronic functional fillers and chemical modification for improving the output performance are further summarized. Meanwhile, the latest cellulose-based hybrid PTENG is also introduced from the fundamental design to the investigations on enhanced strategies. The opportunities and challenges of these cellulose-based nanogenerator devices are put forward in the final part, which could enable this upto-date and state-of-the-art review to be an effective guidance for the future research on cellulose-based nanogenerators in energy harvesting.
(Song, et al., Journal of Materials Chemistry A, 2021)
Developing fibrillated cellulose as a sustainable technological material
Cellulose is the most abundant biopolymer on Earth, found in trees, waste from agricultural crops and other biomass. The fibres that comprise cellulose can be broken down into building blocks, known as fibrillated cellulose, of varying, controllable dimensions that extend to the nanoscale. Fibrillated cellulose is harvested from renewable resources, so its sustainability potential combined with its other functional properties (mechanical, optical, thermal and fluidic, for example) gives this nanomaterial unique technological appeal. Here we explore the use of fibrillated cellulose in the fabrication of materials ranging from composites and macrofibres, to thin films, porous membranes and gels. We discuss research directions for the practical exploitation of these structures and the remaining challenges to overcome before fibrillated cellulose materials can reach their full potential. Finally, we highlight some key issues towards successful manufacturing scale-up of this family of materials.
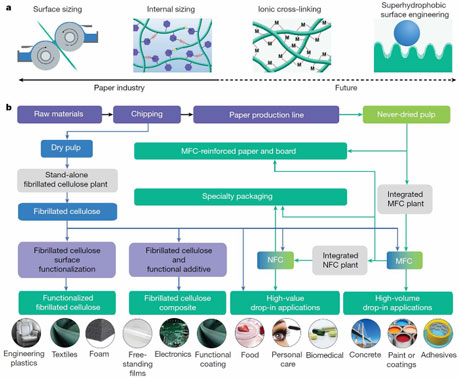
(Li, et al., Nature, 2020)
Analysis of celluloses, plant holocelluloses, and wood pulps by size-exclusion chromatography/multi-angle laser-light scattering
Size-exclusion chromatography with multi-angle laser-light scattering and refractive index detection (SEC/ MALLS/RI) provides the number- and weight-average molar masses, molar mass distributions, conformations, and linear/branched structures of polymers. In the case of pure celluloses including highly crystalline tunicate and alga celluloses, and hemicellulose-rich plant holocelluloses, soaking in ethylene diamine (EDA) and subsequent solvent exchange to N,N-dimethylacetamide (DMAc) though methanol is effective for complete dissolution in ~8% (w/w) LiCl/DMAc. SEC/MALLS/RI analysis can, therefore, be applied to pure celluloses, chemical wood pulps, and plant holocelluloses after dissolution in ~8% (w/w) LiCl/DMAc, dilution to 1% (w/v) LiCl/DMAc and membrane filtration. All pure celluloses and the high-molar-mass cellulose fractions of hardwood and grass holocelluloses have linear and random-coil conformations and various average molar masses and molar mass distributions depending on the cellulose and holocellulose resources. In contrast, Japanese cedar (i.e., softwood) holocellulose and softwood bleached kraft pulp have alkali-stable cellulose/glucomannan branched structures in the high-molar-mass fractions.
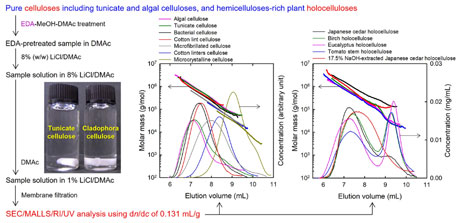
(Ono and Isogai, Carbohydrate Polymers, 2020)
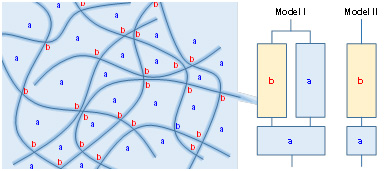
Branched structures of plant cellulose molecules related to plant evolution
Plant holocelluloses can be totally dissolved in 8% LiCl/DMAc after ethylene diamine pretreatment. The plant holocellulose solutions are then analyzed by SEC/MALLS/UV/RI system, to investigate branched structures of high-molar-mass (HMM) cellulose fractions. The results show that the HMM cellulose fractions in hardwood and grasses have the same linear structures as those of bacterial, tunicate, and algal celluloses. In contrast, the HMM cellulose fractions in moss, adiantum, and gymnosperm have branched structures with glucomannan via lignin or lignin-like compounds. These cellulose/glucomannan branched structures may contribute to tall and strong gymnosperm trees with long life times.
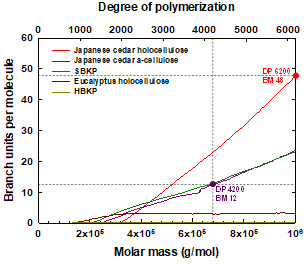
(Ono et al., ACS Symposium Series, 2017)
Xylem Formation in Trees, Analyzed by NanoSIMS/13C-Labelling
High lateral resolution secondary ion mass-spectroscopy (NanoSIMS) in combination with 13C-labelling technique is applied to investigation of the formation process of xylem, consisting of cellulose, hemicelluloses, and lignin. When poplar trees are grown in air containing 13CO2 for short time, distribution of 13C-labelled components in xylem is observed with high resolution by NanoSIMS.
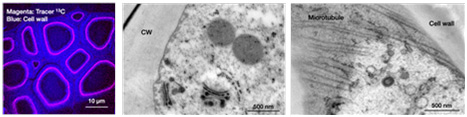
(Takeuchi et al., in preparation)
Cellulose Nanofiber/Polymer Composites and their Characterization
Wood cellulose nanofibers (CNFs) are bio-nanomaterials with high tensile strengths and moduli. Therefore, CNFs are expected as nanofillers for CNF/polymer composite materials with light weight yet high strengths. However, because CNFs are originally obtained as water dispersions and therefore highly hydrophilic, CNFs easily aggregate in hydrophobic polymer matrices during mixing process, resulting that almost no positive properties appear on the composites. In contrast, CNF/elastomer mixtures are mixed under high shear conditions using a three-roll mixer, high tensile strengths, moduli, and ductility concomitantly appear on the composites. STEM and NMR analyses of the composites show that homogeneous distribution of CNFs in polymer matrix is the key factor, influencing the improvement of mechanical properties of the CNF/elastomer composites.
(Noguchi et al., Composite Science and Technology, 2020)
Self-Assembly Behavior of New Cellulose Nanocrystals
Needle-like new cellulose nanocrystals (CNCs) with aspect (length/width) ratios of <50 can be prepared from TEMPO-oxidized cellulose fibers by sonication in water for long time. When dynamic light scattering analysis is applied to CNC/water dispersions with various CNC consistencies, the transition CNC concentration from homogeneous dispersion to gel state can be determined. When the CNC length decreases to 1/2, the gelation point of the dispersion increases to 4 times as much as that of the original TEMPO-oxidized cellulose nanofiber dispersion.
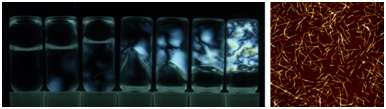
(Zhou et al., Biomacromolecules, 2019)
Preparation of Cellulose Nanofiber/FeOOH Composites for F-Ion Trapping
Iron oxyhydroxide (FeOOH) molecule has a unique structure, in which fluoride ion can selectively be trapped. When FeOOH molecules are formed on the TEMPO-oxidized cellulose nanofibers as templates, the FeOOH/cellulose nanofiber composites are expected to efficiently remove fluoride ions for drinking and industrial water grade.
(Umehara et al., in preparation)
Determination of dn/dc value of Cellulose in 1% LiCl/DMAc
When weight- and number average molar masses of cellulosic materials are determined by SEC/MALLS, an accurate dn/dc value of cellulose in 1% LiCl/DMAc used as an eluent should be determined beforehand. In literature, various dn/dc values have been reported, and consequently the calculated molar mass values vary, depending on the dn/dc value. We have made clear the reasons for discrepancy of dn/dc values between publications, and an accurate dn/dc value is obtained as 0.131 mL/g for cellulose in 1% LiCl/DMAc. Because each hydroxy group of cellulose forms an unstable complex structure with LiCl and DMAc, the dn/dc values change, depending on the cellulose concentration. Furthermore, dissolution states of chitin, pullulan, and other polysaccharides and their derivatives in solutions have been clarified, associated with their accurate dn/dc values, by SEC/MALLS.
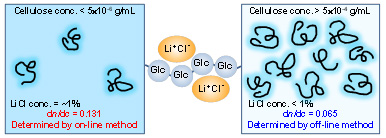
(Ono et al., International Journal of Biological Macromolecules, 2018; Biomacromolecules, 2018)
Distribution of Carboxylate Groups in TEMPO-Oxidized Celluloses
Distributions of carboxylate groups in cellulose molecules, which are introduced to cellulose fibers by TEMPO-mediated oxidation, are analyzed by position-selective methyl and methyl-anthracene esterification, and successive dissolution in LiCl/DMAc and SEC/MALLS/UV/RI analysis. In the case of algal cellulose microfibrils with a large crystal size, carboxylate groups are formed selectively in the cellulose microfibril surfaces associated with partial surface-depolymerization. In contrast, in the case of TEMPO-oxidized plant celluloses, carboxylate groups are homogeneously distributed from low- to high-molar-mass regions, indicating that each plant cellulose microfibril has a different cellulose chain packing structure from that of algal cellulose.
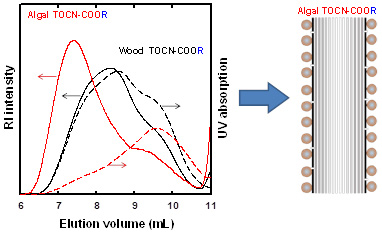
(Ono et al., Biomacromolecules, 2019)
Structural Analyses of Plant Cellulose Microfibrils
Wood cellulose fiber is oxidized by TEMPO-mediated oxidation and successive sonication in water. The kink formation and fragmentation mechanisms of the obtained TEMPO-oxidized cellulose nanofibers and nanocrystals are analyzed by AFM, SEC/MALLS, and solid-state 13C-NMR analyses. The results show that each wood cellulose microfibril consists of 32 fully extended cellulose chains in average, forming a hexagonal cross section structure. Furthermore, TEMPO-mediated oxidation causes not only position-selective oxidation of C6-OH groups to C6-carboxylate groups but also significant side reactions such as depolymerization, partial detachment of surface chains, and formation of homopolymer chains and disordered regions occur simultaneously, which cannot be ignored in TEMPO-mediated oxidation of natural cellulose fibers.

(Zhou et al., Biomacromolecules, 2020)
Related Analytical Apparatuses

CAMECA NanoSIMS 50L
(MEXT project)

JEOL solid-state CP-MAS 13C-NMR
(JST-CREST project)

MOCON oxygen and moisture permeability analyzers
(NEDO Nanotech project)

SEC/MALLS/UV/RI system
(JSPS project)

High-performance liquid chromatogram for sugar composition analysis(JST-CREST project)

Ultrasonic homogenizer

Dynamic laser particle size/
zeta-potential analyzer

High-pressure homogenizer

Phase-contrast optical microscopy

Electron spin resonance apparatus

pH stat (automatic pH controller)






